Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Common overlooked costs when calculating Medical Device manufacturing costs. Non-BoM costs of producing devices can add up quickly.
-

Pros and Cons that help determine when to switch to CAD– How soon is too soon? Medical device examples and three sanity checks.
-

10 Program Kickoff tips to consider for a great program start to stay on track with time, budget and expectations.
-

3 Tips for defining the overall critical path (and critical path bottlenecks) for medical device project success. Do not underestimate!
-

Recent report on policies and initiatives claims the FDA device arm (CDRH) medical device approval process is much faster than five years ago.
-
Spinal care devices highlight the future of digital health. Innovations based on digital information shared in the healthcare network will drive adoption.
-
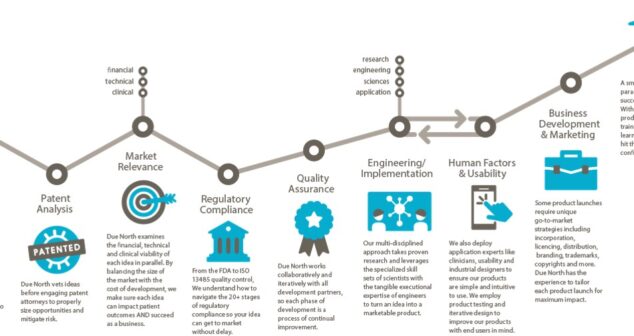
Sketch to Launch commercialization process for medical devices guides translational services to the medical industry.
-

How a mock FDA inspection led us to learn the secret to closing open CAPAs by 50%. Vesna Janic shares the story and her tips.
-

Advice, implications and examples for best time to implement a medical device Quality Management System in US, Canada and Europe.