Recently, I read an FDA report entitled “Understanding Barriers to Medical Device Quality”, which examines the current quality status of medical devices. This informative report compiles the serious adverse events recorded in FDA’s Manufacturer and Used Facility Device Experience (MAUDE) databases, the Recall Enterprise System- ORA database (RES), as well as interviews with FDA officers, industry leaders and other research experts. The report uncovers many interesting facts about the marketed medical device industry, including:
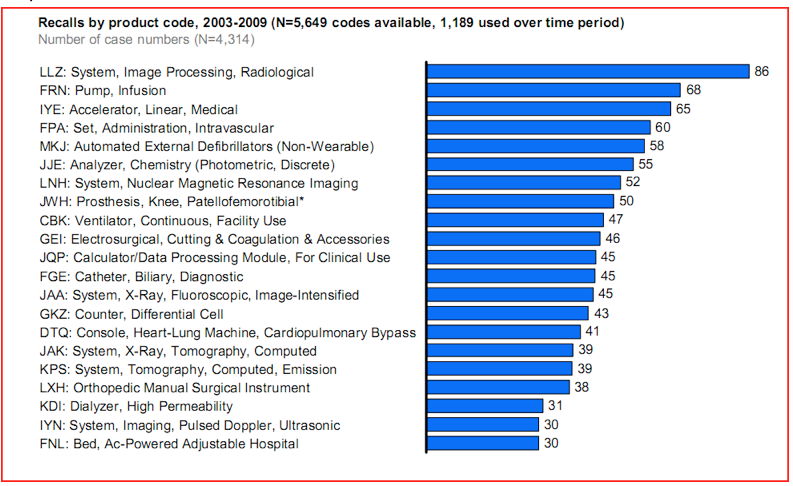
Quality risk distribution is uneven. While the most frequently recalled products are radiology devices, 60% of adverse events are related to cardiovascular devices , in vitro diagnostic (IVD) and general hospital/surgical devices combined.
This industry has experienced significant growth in both revenues and technical complexity.
The increase in serious adverse events has outpaced industry growth by 8 % since 2001.
- Root cause analysis reveals that more than 50% of recalls are due to product design and manufacturing process control failures. Hardware issues are significant contributor to product recalls.
- Based on their analysis, The FDA outlines the following opportunities for improvement:
- Improved design and reliability engineering (emphasis on validation, manufacturability and software robustness);
- More robust post postproduction monitoring and feedback;
- More stringent supplier management, focusing on material and process change control;
- Strengthening quality metrics and measurement systems;
- A move towards cross- functional quality organizations. For example, design engineers should not be measured only on ‘time to market’ but also on the actual quality of the product and/or manufacturing processes;
- Accountability and performance management- key roles should be associated with quality outcomes;
- Stronger quality culture, with performance incentives for all levels based on fulfilling quality goals.
Understanding Barriers to Medical Device Quality also features several industry recommendations to the FDA. These include featuring high-quality manufacturers as “case studies” as a learning tool for small medical device companies, more quality review initiatives similar to the one conducted in response to sector-wide infusion pump quality failures, and improvements in enforcement consistency and transparency on behalf of the FDA.
Recommendation
Understanding Barriers to Medical Device Quality was interesting and informative. I liked the emphasis and suggestions for establishing a strong quality culture. I strongly recommend this report to any small or large medical device manufacturing company.
Vesna Janic is the Director of Quality/Regulatory at StarFish Medical. She uses her expertise in quality systems and regulatory compliance to guide our QA/RA team and help clients avoid wasting time on their path to the market.