
StarFish Medical Services Overview
Medical Device Product Engineering Services with StarFish Medical
From idea to Market-Leading device
Unlock the Full Potential of Your Medical Device Development
Overcome the Complexities of Medical Device Development
Developing medical devices is a challenging journey fraught with regulatory hurdles, technical complexities, and market demands. The path from ideation to commercialization requires not only innovative thinking but also meticulous planning and execution. In an industry where safety, efficacy, and compliance are paramount, having a trusted partner can make all the difference.

Experience Comprehensive Support and Expertise
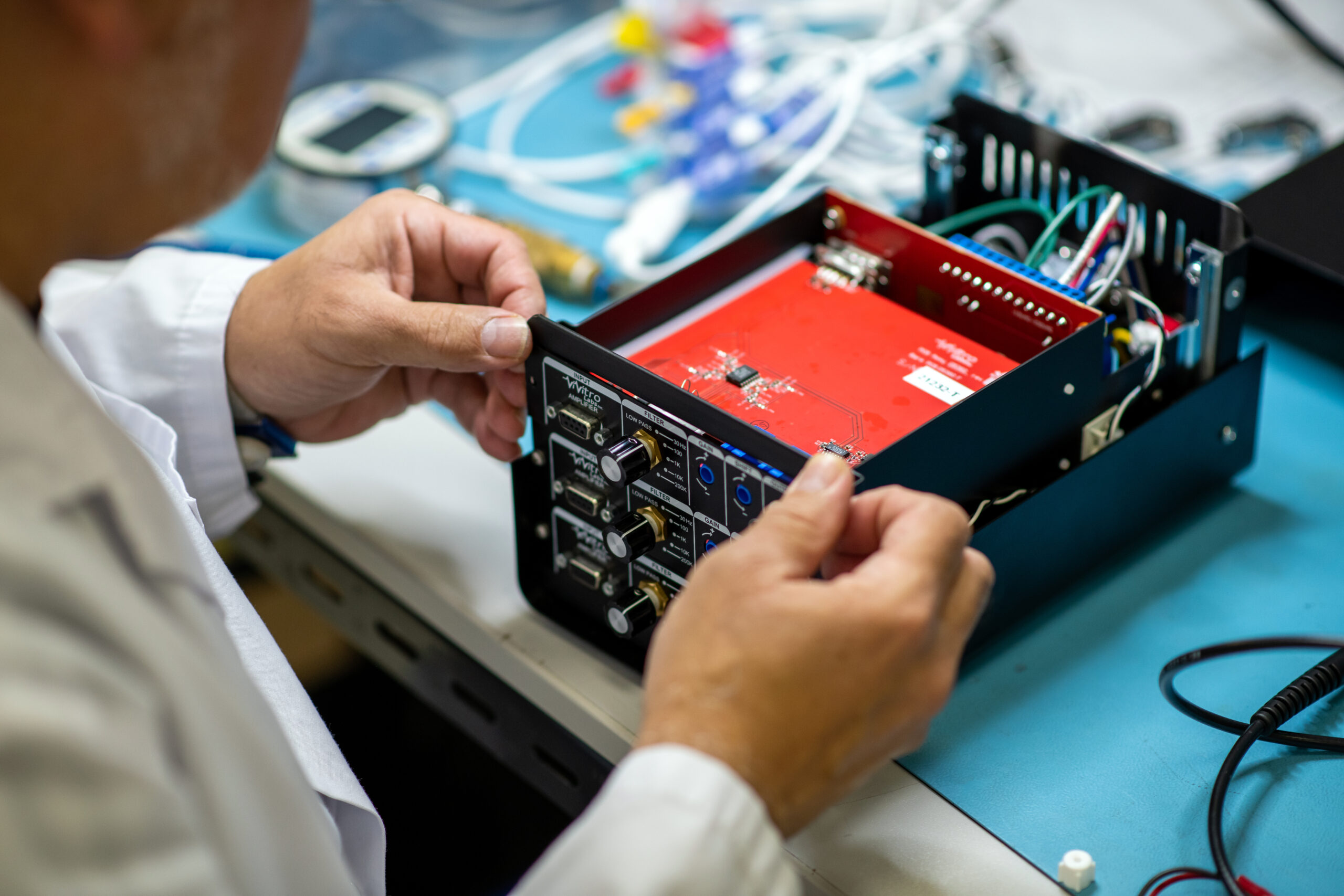
At StarFish Medical, we specialize in guiding medical device companies through every stage of the development process. Our multidisciplinary team of experts is dedicated to transforming your vision into a market-ready product. From initial concept to high-volume production, we provide comprehensive support to ensure your success.

Product Design & Development – Transform Your Vision into Market-Ready Products
Custom product design and development from concept to final product
You’ve got the vision. We help you bring it to life. StarFish Medical offers full product design and development medical device product engineering services that propel your concepts from initial idea to final market-ready product. With a multidisciplinary team of experts dedicated to your project’s success, you’re assured a final product that meets the highest standards of safety, efficacy, and user experience.
Key Services:
- User needs analysis
- Product conceptualization
- Feasibility studies
- Rapid prototyping and testing

New Product Introduction Ensure a Smooth Transition to Manufacturing and Launch
Develop a product that’s ready for manufacturing and commercial launch
Prepare to launch. Transform your vision into a safe, functional product that meets a defined market need. StarFish Medical employs cutting-edge NPI strategies to deliver products that truly serve patients and clinicians. By performing all medical device product engineering services activities in-house, we ensure a streamlined, lower-risk process when transitioning to full production.
Key Services:
- Supply chain development
- Vendor qualification
- Regulatory clearance preparation
- Design for manufacturing (DFM) principles
Quality Assurance and Regulatory Affairs – Navigate Regulatory Challenges with Confidence`
Complex regulatory path?
We know the way.
Navigating the journey from product design to regulatory clearance is no small feat. The path is riddled with obstacles that can delay your time to market and increase risks. To confidently steer through these complexities, you need a partner with deep expertise and a proven track record.
Key Services:
- Regulatory strategy and submissions
- QMS design and implementation
- Mock audits and independent reviews
- Clinical and product submissions

Chart your course with expert regulatory affairs consulting.
Partner with StarFish Medical
With over 25 years of experience, StarFish Medical is your trusted partner in medical device development. Our integrated approach to medical device product engineering services ensures that every aspect of your project is handled with the utmost care and expertise. Let us help you navigate the complexities of medical device development and bring your innovative solutions to market.