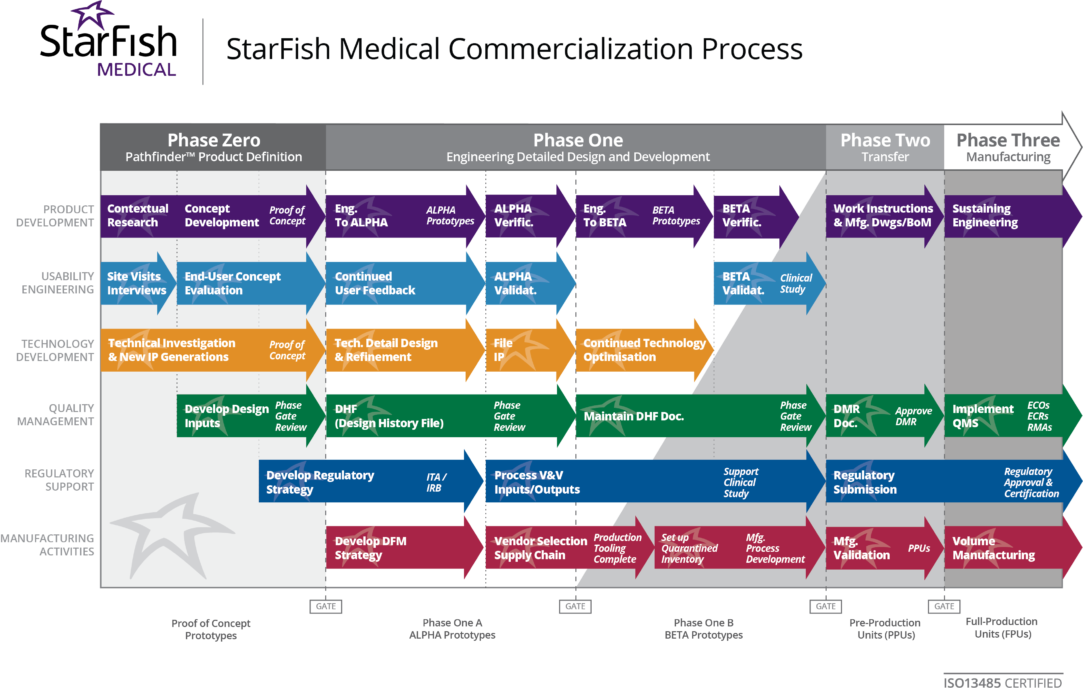
Design and Development Process Improvement
Whether you are thinking about Annual Planning or Year End Closing, you’re in a time of reflection and commitment to improvement. Over the years I’ve learned to choose a limited number of improvement efforts, apply management resources, then measure with them regularly for progress and impact. The costs of not focusing sufficiently in a few key areas can ruin a great idea in complex Enterprise Partnership projects.
Here are my five top Medical Device Design and Development Process Improvement recommendations for your Annual Plan:
1. Get Plenty of Clinical Input. Building a useful medical device means understanding clinical workflow, the reaction of clinicians, and the ergonomics of surgery well enough to make choices and decisions that will work in a real-life work environment. Spend enough time watching the procedure you are trying to improve before making any changes or spending product development resources. Annelies Tjebbes’ recent blog on Innovation and Value raises great points from a user’s perspective.
One of my favorite examples of a failure to get sufficient clinical input involves an intravascular ultrasound system. The second generation model featured a trendy new touchscreen to replace a tried and true tactile operator interface. Unfortunately, operators reverted to the old model despite other disadvantages because they hated interacting with the new, “advanced” touchscreen.
2. Worry About the Financial Model Pay as much attention to the financial model and margins for a product as the technology. Think beyond the cost of technology. Consumables, distribution in the field- especially for novel devices in medical specialty areas, manufacturing and supply chain management all add significantly to the raw cost of goods. Carefully consider any reimbursement model as well as the cost and time required to get a new Current Procedural Terminology (CPT) code.
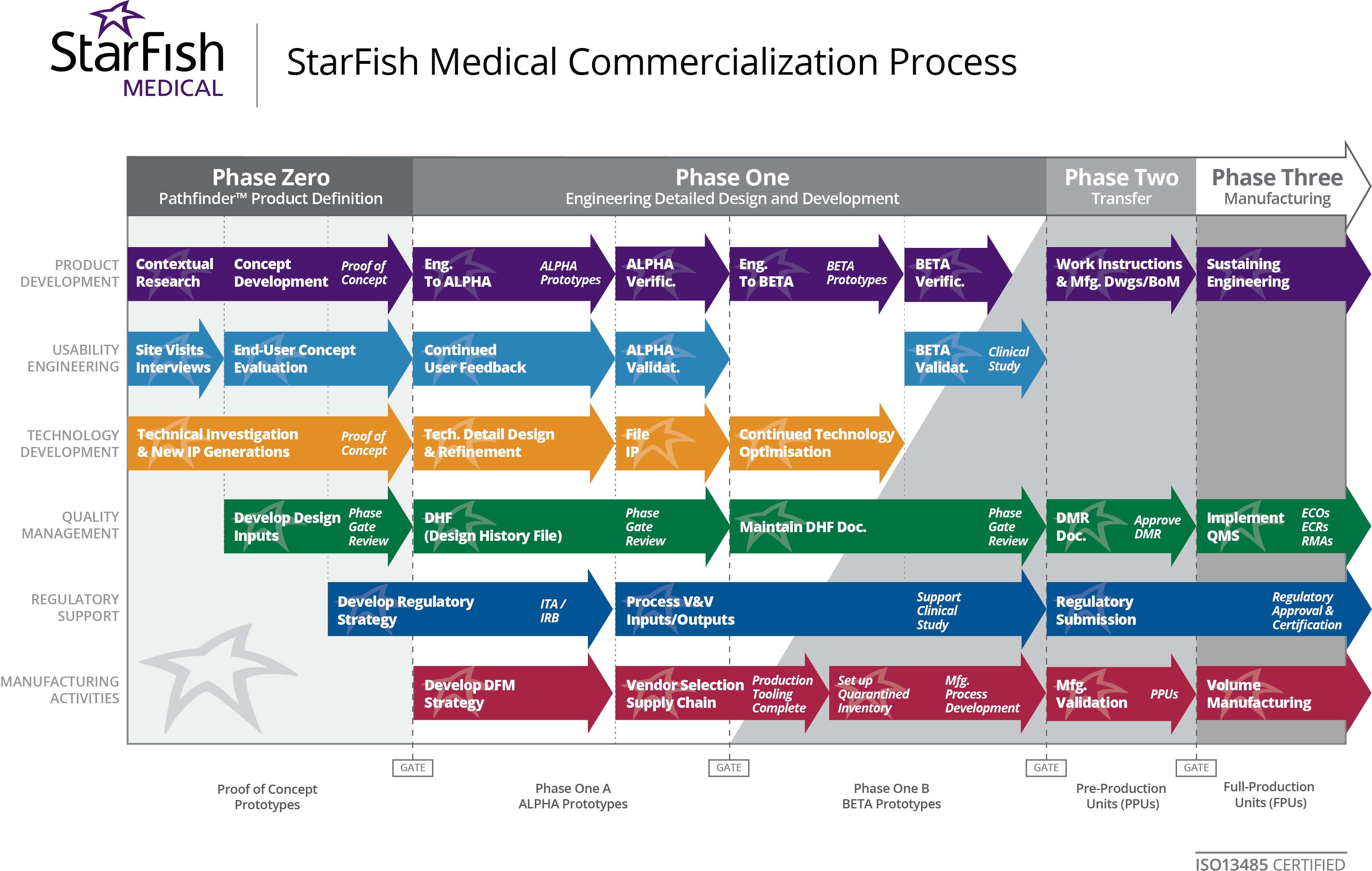
3. Document Critical Requirements In many cases companies miss critical requirements by not documenting them or by not being aware of changes at the FDA. According to ISO13485 and FDA QSR, specifications are supposed to be driven in part by a risk analysis. Often companies skip that step and end up having to layer risk analysis in afterwards which defeats the purpose and exposes them to expensive re-spins. Other typical examples would be required standards such as IEC60601 for safety, HE75 for human factors engineering, and ISO62304 for medical software, or ISO13485 for the quality system.
4. Think About Regulatory Early The risk class of a medical device often determines how much clinical data will be required for Regulatory Clearance. Do not underestimate the impact. Regulatory clearance can be more expensive than the whole product development effort. A product can be redesigned to drop it down in risk by, for example, making motorized actions manual. Because it is one of the highest risk areas in most development programs, make sure it gets a lot of early attention. Often projects get quite far advanced before the risk class is figured out and the financial impact is huge.
5. Make Enough Prototypes Everybody inside a company already believes in their new medical device and that prevents them from seeing the obvious usability and functional issues in the prototypes. They often do not understand competing IP well enough. That’s why making and sharing prototypes make my Top 5 list. Many times I’ve seen situations where expensive redesigns had to be undertaken to resolve fundamental functional issues which could have been bench tested before expensive detailed product design was undertaken. While companies are understandably concerned about their ideas getting out into the public, they expose themselves to additional risk by not getting outsiders to look at and try their prototypes.
Next Steps
Follow these Design and Development Process Improvement recommendations and your Medical Device Development Annual Plan will be on the way to a banner year. I’d love to hear from readers (and talk to them) about how they are improving their design and development efforts in their annual planning.
Scott Phillips is Founder and CEO at StarFish Medical. Please visit our Services page for more information on how we address Medical Device Design and Development and work with Startups & Founders at StarFish Medical.
Images: StarFish Medical
Join over 6000 medical device professionals who receive our engineering, regulatory and commercialization insights and tips every month.