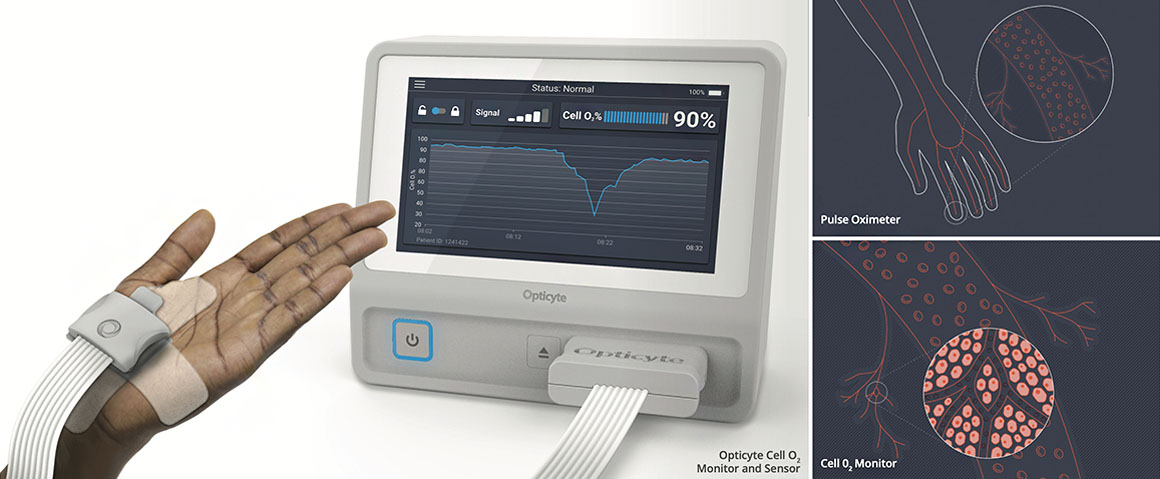
Opticyte Cell O2 monitor measures oxygen inside cells in real-time
Measuring oxygen levels inside cells, the Opticyte Cell O2 monitor enables clinicians to detect and diagnose organ dysfunction in real-time and make optimal treatment decisions for their critically ill patients.
Founded in 2016, Opticyte was spun out of the University of Washington. Having secured more than $4.0M in non-dilutive funding, this medical device start-up is transforming the standard of care for patients at risk of organ failure and death due to sepsis, with the industry’s first real-time Cell O2 monitor.

“I really value the team at StarFish.”
Lori Arakaki, Co-founder and CEO, Opticyte
Challenges
Early prototypes developed in the Opticyte lab were too large and expensive to be a viable product, so they needed a way to reduce the device to a stationary monitor and a single use sensor that could be easily and affordably manufactured. Opticyte engaged StarFish Medical to help them bring this smaller, more affordable Cell O2 monitor from concept stage to a prototype used to study hospital patients.
Results
“One of my biggest concerns about hiring a third-party firm to do our engineering work is that we wouldn’t have all the control over the timelines and the progress. Having StarFish be our firm instead of hiring our own engineers in-house has been a wonderful solution for us. Our program manager completely understands what’s needed technically for success, but he also understands how to support us as a small business, as a startup.”
“We have been able to be a very capital efficient startup, which is super important for us founders, but also for early investors, because we’ve made a lot of progress. We’ve accomplished so much with relatively little dilutive capital.”
“I really value the team at StarFish. Every person on our team has evident clinical expertise. That is very valuable. They’re all professional. Great to work with. They are fun to work with. StarFish covers all of the different kinds of engineering that we need, whether it’s electrical engineering, mechanical engineering, industrial design, and even help with our regulatory path. And I appreciate all of that very much.”
Bottom Line
The Opticyte Cell O2 Monitor is a $7 Billion+ Global Market Opportunity. The Cell O2 Patient Monitor received Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) which provides for timely interactions with the FDA and prioritized review of the regulatory submissions. Opticyte has been capital-efficient and is funded for the next year with a non-dilutive grant. The company has issued patents, a clear De Novo pathway, and the potential to deliver significant cost savings to hospitals. Demonstrating strong momentum and credibility, Opticyte is a medical device innovator to watch.
The company is currently raising seed funding. Milestones include product development, FDA clearance, and execution of a multi-center randomized controlled trial.