Immunoassays are the heart of most bioanalytical techniques used in clinical and research settings, allowing for accurate detection and quantification of biomolecules. Transferring an immunoassay to a point-of-care[1] system can be a challenging process. The traditional ELISA approach often requires multiple timed incubation steps, trained personnel, and expensive equipment with long delays from sample to answer. A Modular Platform for transferring immunoassays speeds development time.
The detection and quantification of an analyte is achieved using capture reagents, usually antibodies or antigens. The binding interactions between the analyte and the capture reagent are highly sensitive and specific, allowing for accurate and precise measurements.
What inspired the development of the modular platform for transferring immunoassays ?
Recent advancements developing immunoassays that can be deployed at the point of care. Nonetheless, ensuring accuracy and precision in assay transferability remains a crucial challenge. But what if there was a versatile solution that could ease that transition and be modular to different assays? Introducing a modular platform that combines a customizable fluidic actuation, reagent storage, detection method, and interface with equipment allows the creation of a versatile solution as an intermediate bridge between assay development and a portable POC (Figure 1).
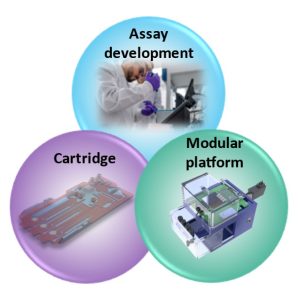
Overview and requirements of the modular platform for transferring immunoassays
Before developing the modular system, let’s take a step back and re-examine the steps needed to transfer an immunoassay to a disposable cartridge. Before diving into the details, it is essential to ask questions like what the workflow for the assay looks like, i.e., competitive or Sandwich ELISA, what type of detection method is being used – optical or electrochemical, and what kind of sample is being tested. Additionally, you should consider whether any sample pre-processing or reagent mixing is necessary and what quality control measures are required. Once these questions are figured out, the system can be adapted to the specific requirements of the assay.
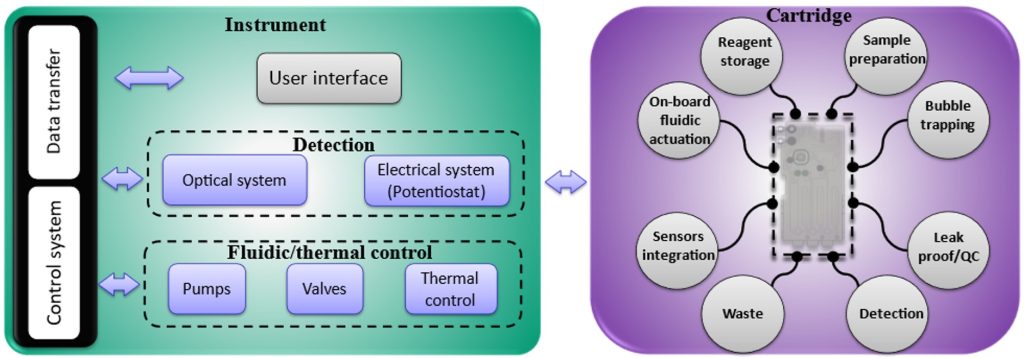
The platform (Figure 2), consisting of an instrument and a cartridge, was created to be readily adaptable to address all these queries, some managed by the instrument part and others incorporated into a modular cartridge. The instrument part takes care of fluidic actuation through integrated pumps, solenoid valves, and bubble trappers; detection through integrated potentiostat or attaching it to a microscope; and communication through USB. The modular cartridge incorporates valves, a reaction chamber, a mixing stage, waste handling and different reagent storage and introduction techniques. It also interfaces with the sample preparation cartridge and the target surface, i.e., pre-functionalized and antibody-coated screen-printed electrodes or glass slides.
Considerations when designing the modular cartridge.
The cartridge is a vital component in the point-of-care system, yet determining the appropriate form factor for the cartridge is challenging. It requires a series of trial-and-error processes to achieve proper dimensioning, adequate mixing, material selection, reagent compatibility, and efficient bonding. Our modular cartridge (Figure 3) has eliminated these challenges from the development process by considering the following:
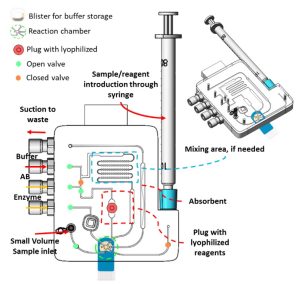
- Reagent storage and sample introduction
The complexity of the assay determines which reagents should be introduced or stored on the cartridge. The cartridge is designed to accommodate up to five (5) different reagents using various methods, including blister packs, plugs containing lyophilized reagents, lyophilized beads, a liquid tank, and a syringe inlet. It can process various samples, including blood, saliva, urine, and other bodily fluids. The cartridge can adjust to the sample’s volume, ranging from µL to mL, by optimizing channel dimensions and fluid flow and minimizing dead volume.
- Mixing
In specific assays, like competitive ELISA, mixing the sample with a known target analyte concentration may be necessary before the enzyme can further bind with it. For this purpose, a mixing module is included in the cartridge and can mix two separate reagents by activating the integrated valves.
- Fluid actuation
To enable modularity, fluid actuation is achieved via mp6 micropumps or vacuum pumps. These pumps allow precise and accurate fluid actuation, resulting in reliable and consistent results. A bubble trapper is also integrated into the system to remove air bubbles when necessary.
- Optical or Electrical Detection
The reaction chamber, a cartridge’s sensing region, is where the binding interaction and the detection happen. It is designed to be connected to pre-functionalized and antibody-coated surfaces, such as screen-printed electrodes for electrochemical detection or glass slides for colorimetric detection. This technique allows for easy swapping of the electrodes/glass slides while using the same cartridge for multiple assays. For electrochemical detection, a PalmSens Emstat potentiostat is embedded within the system for direct detection. On the other hand, the instrument fits within a custom-built microscope for optical assays.
- Waste handling
The cartridge includes absorbent pads and waste containers that capture any waste generated during the process.
Overall, the modular platform for transferring immunoassays acts as an intermediate bridge between assay development and the final point of care. It is a significant step towards streamlining the product development process for diagnostics. The platform’s modularity enables clients to reduce the time and resources required to verify the assays and develop new cartridge-based diagnostic devices.
[1] Per ISO 22583:2019 and ISO 15189:2022, testing that is performed near or at the site of a patient.
Image: StarFish Medical
Khaled Youssef is a Bio Services Engineer at StarFish Medical with an academic background that includes a BSc and MSc in Mechanical Engineering from Egypt, a Ph.D. in Mechanical Engineering, and a graduate diploma in Neuroscience from York University. His work has covered the development of microfluidic devices for neural and behavioural screening of living organisms, innovative materials for health and safety applications like smart bandages for chronic wounds, microspheres for bacterial isolation from water sources, point of care diagnostics development, and tissue engineering. Khaled’s research and R&D experience resulted in over 30 publications (hindex=10) and one patent. At StarFish Medical, Khaled applies his engineering and life sciences expertise to support Bio Services projects in different capacities, including microfluidics, systems, and mechanical engineering, and as a research scientist