Optical imaging tools can enhance the crucial role of biomedical imaging in accurately identifying diseases and monitoring their progression.
Conventional medical imaging devices such as X-rays, computed tomography (CT), magnetic resonance imaging (MRI), ultrasound, and positron emission tomography (PET) are commonly used in clinical settings (Figure 1). However, these imaging modalities are limited in their resolution, only being able to capture images at the millimeter scale or greater.
To achieve an accurate diagnosis, higher resolution examination of cellular structures through a biopsy may be necessary. The integration of optical imaging with conventional imaging techniques could be a more effective approach to address this need.

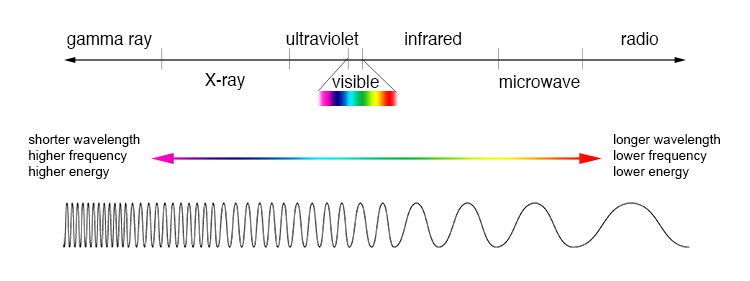
Optical imaging, which utilizes light in the visible to near infrared spectrum (Figure 2), offers several advantages over conventional high-frequency imaging. Optical imaging can achieve image resolutions as small as a few microns, allowing for cellular-level visualization, and does not use ionizing radiation or high-frequency sound waves.
Specific molecules or cells within a tissue can be targeted for imaging or analysis. These optical imaging modalities complement conventional imaging ones, providing a more comprehensive picture of biological processes and systems and ultimately reducing variation and time to results [1].
Although optical imaging offers higher resolution than conventional imaging, the imaging depth is limited to a few millimeters into tissue due to the absorption and scattering of light as it passes through biological systems. Tissue transparency depends on factors such as composition, density, thickness, and hydration level. Tissues are composed of cells and vessels that present changes in the refractive index to light, resulting in light scattering or simply a change in the direction of the light.
Attenuation of light occurs in the presence of light-absorbing tissue components called chromophores, such as hemoglobin and melanin. Both scattering and absorption are wavelength dependent and affect how deeply light can penetrate into tissue [2].
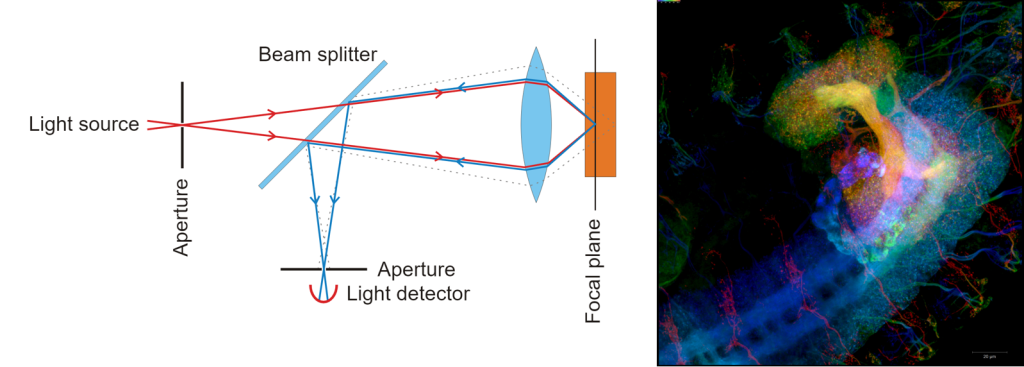
Various optical imaging modalities have been developed to aid clinicians and researchers in the interrogation of biosystems [3]. Confocal microscopy, nonlinear microscopy, and optical coherence tomography (OCT) are a few examples. Confocal microscopy rejects out-of-focus light from the detector via a pinhole, providing higher resolution images over traditional widefield microscopy imaging of thick samples (Figure 3).
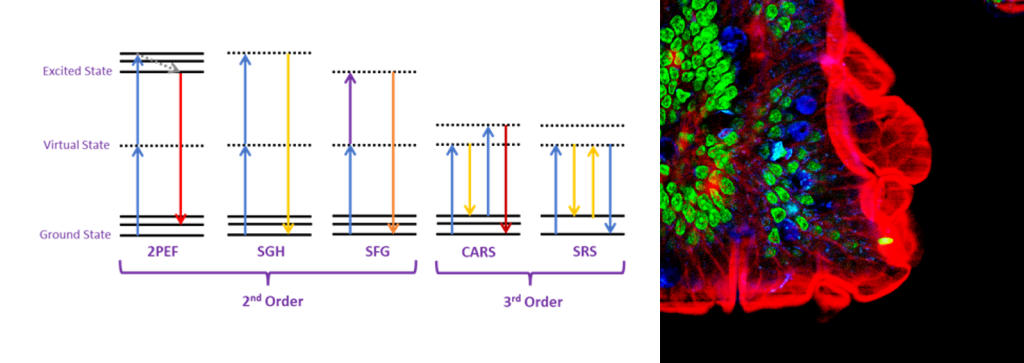
Nonlinear microscopy allows for label-free and molecular-specific imaging of biological processes in cells and tissues. The multi-photon nature of these imaging processes results in increased resolution of the imaging and reduced out-of-focus photobleaching (Figure 4).
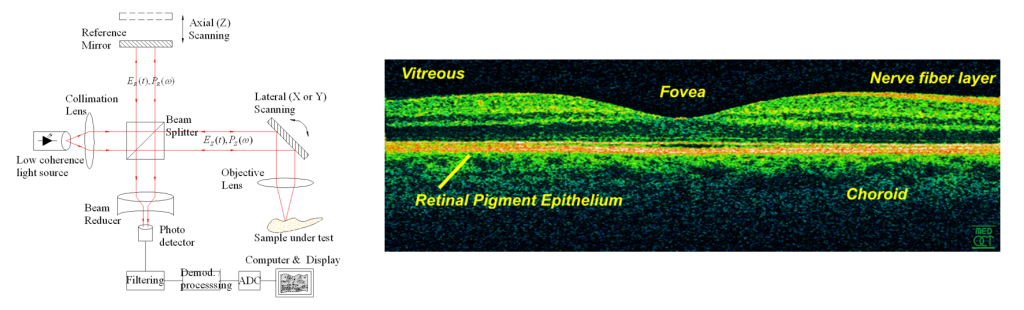
OCT derives a signal from the low-coherence interference between the reference beam and the scattered back-reflected light from the tissue, allowing for the formation of 2D and 3D images (Figure 5).
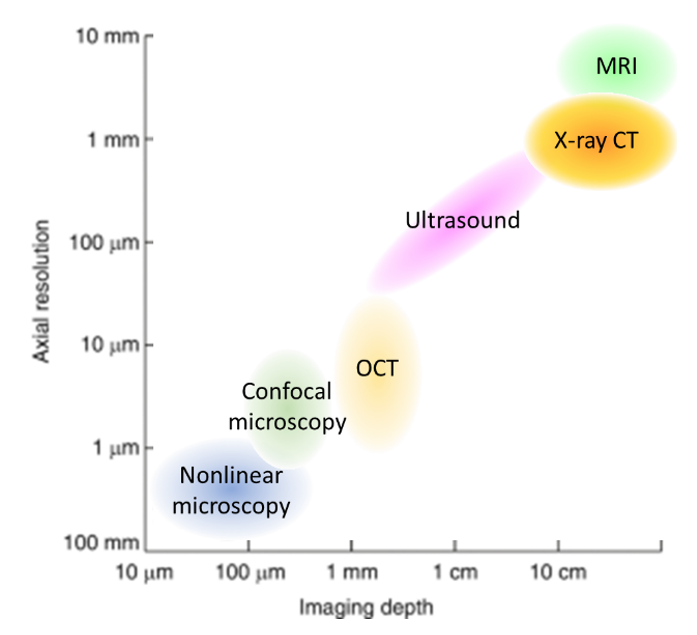
Optical imaging methods are predominantly used in ex vivo environments or in vivo if the tissue of interest is easily accessible and superficial. Combined imaging modalities could provide enhanced information for clinicians on both macro and micro scales (Figure 6), leading to well-informed decisions more quickly.
To make optical technologies more relevant in the clinical environment, the issue of imaging depth must be addressed. One promising development that addresses the imaging depth is the delivery of optical imaging via optical fiber probes for minimal invasiveness.
Biomedical imaging is a valuable tool used to create visual representations of biological processes, providing important information for clinicians and researchers. Advancements in optical imaging technologies offer the potential to improve healthcare outcomes and advance scientific knowledge – the future of clinical imaging looks bright.
Sources
Figure 1: https://www.freepik.com/free-vector/medical-examination-icons-set_4411890.htm#query=xray%20icon&position=0&from_view=keyword&track=robertav1_2_sidr
Figure 2: https://imagine.gsfc.nasa.gov/Images/science/EM_spectrum_compare_level1_lg.jpg
Figure 3:
Left – https://commons.wikimedia.org/wiki/File:Confocalprinciple_in_English.svg#filelinks)
Right – https://www.flickr.com/photos/zeissmicro/26491464051/in/photostream/ )
Figure 4:
Left – StarFish Medical
Right – https://commons.wikimedia.org/wiki/File:MultiPhotonExcitation-Fig10-doi10.1186slash1475-925X-5-36.JPEG
Figure 5:
Left – https://commons.wikimedia.org/wiki/File:OCT_B-Scan_Setup.GIF
Right – https://commons.wikimedia.org/wiki/File:Retina-OCT800.png
Figure 6: StarFish Medical
References
[1] National Institute of Biomedical Imaging and Bioengineering (NIBIB), “Optical Imaging,” [Online]. Available: https://www.nibib.nih.gov/science-education/science-topics/optical-imaging. [Accessed 28 April 2023].
[2] Hochschule Koblenz University of Applied Sciences, “Introduction to Tissue Optics,” [Online]. Available: https://www.hs-koblenz.de/mut/forschung-projekte/labore-projekte/labor-fuer-biomedizinische-optik/forschung/introduction-to-tissue-optics. [Accessed 24 April 2023].
[3] Yashin et. al., “Editorial: Optical Imaging and laser technologies in neuro-oncology,” [Online]. Available: https://www.frontiersin.org/articles/10.3389/fonc.2022.1103711/full. [Accessed 21 April 2023].
Tammy Lee is an optical systems engineer. She has more than a decade of commercial experience with research and product development of optical systems. She holds a Ph.D. in Optics from the University of Rochester.