How Regenerative Medicine will transform therapeutic medical devices
50 years of research in stem cell biology, immunology, molecular biology, genomics, engineering and advanced materials, are transforming the therapeutic landscape much like antibiotics once did for infectious diseases. Regenerative Medicine (RM) and advanced therapeutic products encompass a very diverse and broad range of approaches that enable the body to repair, replace, restore and regenerate damaged or diseased cells, tissues and organs.
At the center of most of these therapies are living cells. For many of these therapies, there is generally a complex multi-step manufacturing process that includes an expansion step to generate sufficient modified cells required for a dose. Complexities in cryopreservation, transport and logistics, in process and release testing, as well as regulatory and reimbursement challenges, must be addressed when developing a RM product.
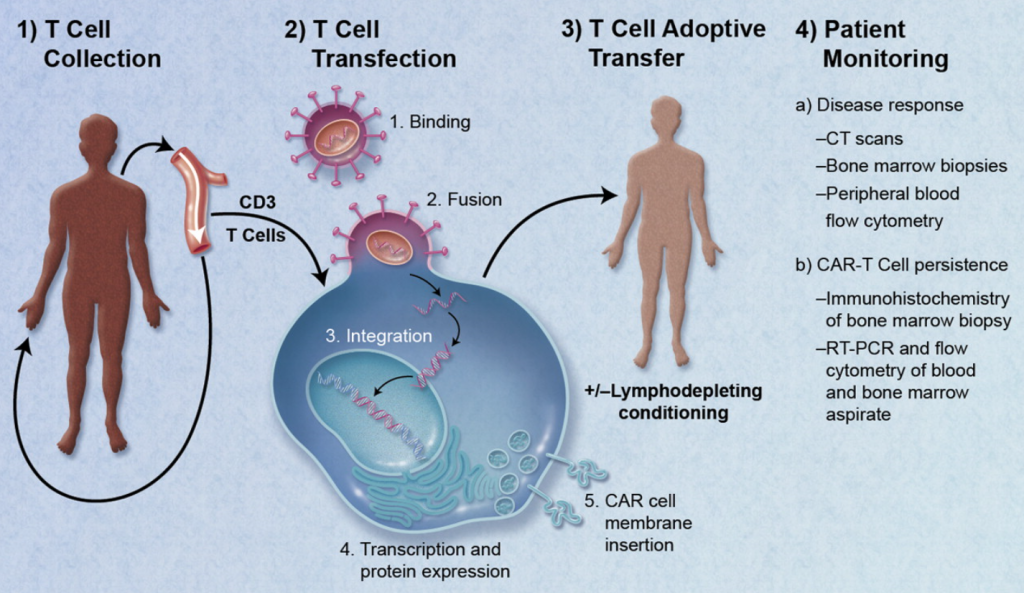
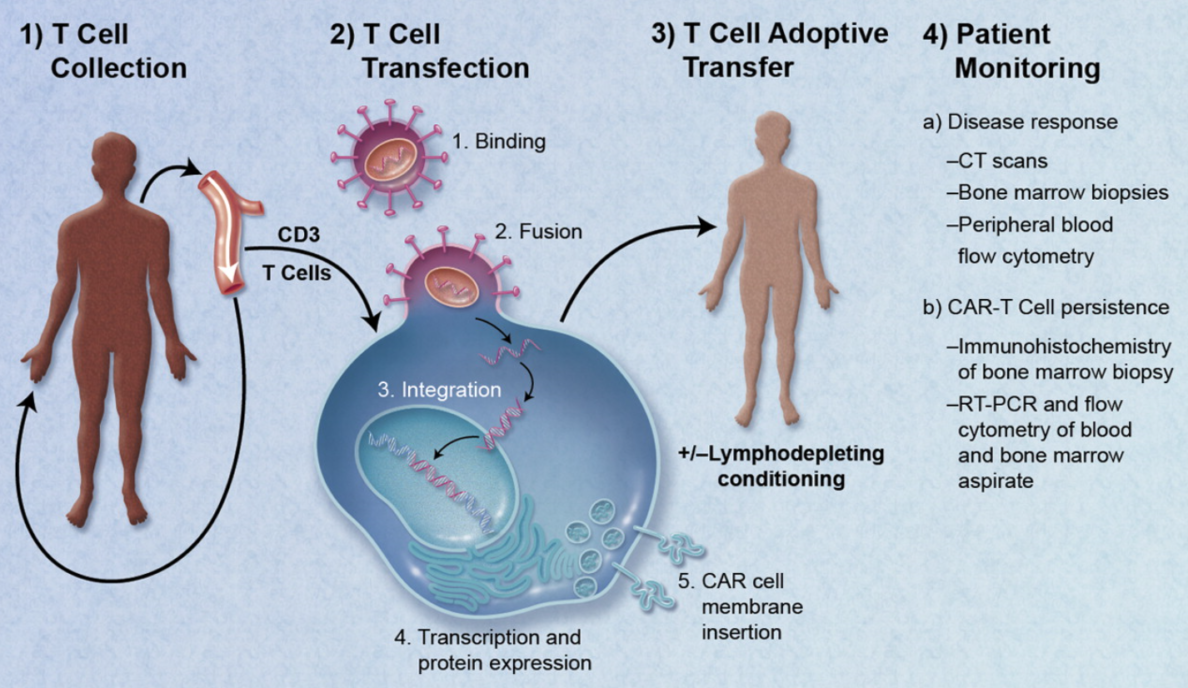
Much of the last 20 years has focused on proving the mechanism of action for these therapies, translation into the clinic, and more recently on cost effective manufacturing processes. The industry is rapidly moving forward. Three therapies gained FDA approval at the end of 2017 and as many as 40 gene therapies might win approval by 2022 from a pipeline of more than 900 development candidates.
Implications for medical devices
Medical devices have not played a critical role in the RM industry to date. The focus has been on the mechanism of action and manufacturing challenges. Medical devices have, however, been an important element, especially in the collection of the starting material and in administration of the final product. Examples for cell collection include apheresis machines and Bone Marrow Aspiration Needles. For administration, most current RM products utilize devices at the simpler end of the spectrum such as syringes, bags and infusion pumps.
The role of Medical Devices in the RM industry is changing as the next generation of products are developed. Two key aspects are the critical role of administering therapies (especially the more complex tissue engineered products) and the game changing approach for near patient processing (NPP) devices.
For many Cell and Gene Therapies, especially where the indication is a hematological cancer, the mode of administration is relatively straightforward. As therapies are developed to treat specific tissues (spine, CNS etc.) with combination products such as tissue engineering approaches that include cells, biodegradable scaffolds, advanced materials and nanomaterials, the administration approach will become more complex and likely require a medical device.
In order for the therapy to be effective, it will require that the correct number of cells are positioned precisely and the cells are viable and potent. This aspect of providing the RM product needs to be explored and understood in sufficient detail early in development, particularly the regulatory framework. This will help to ensure that clinical trials are performed with devices that give the therapy the best opportunity to demonstrate efficacy.
Near patient processing opportunities
With such a wide range of products making up the Regenerative Medicine industry, not all lend themselves to the option of NPP. One size does not fit all. From a business perspective, the two critical elements that would support the NPP approach are the number of patients to be treated and the complexity of the cell manufacturing process.
The annual volume (number of patients to be treated) for a particular indication will significantly influence the cost of any device and associated disposable. If there is a common unit process that is used for many therapies (e.g. collection of the starting material by Apheresis), then the annual volume will be high and this will aid in bringing disposable costs down. On the other hand, the device and disposable cost will inevitably be high for one-off processes for a small indication. Manufacturing methods (such as injection molding) drive down part and manufactured costs for disposable elements, but are only a viable option when the number of units justifies the investment.
The simpler the cell manufacturing process is, the more likely it can be adapted to NPP. Existing approved Stem Cell therapies have shown promise and are potential precursors to NPP solutions of the future. It is important to note that the use of adult mesenchymal stem cells is not FDA approved for the treatment of any specific disease in the United States at this time and their use is therefore investigational.
These approaches have generally focused on the collection and the injection of adult mesenchymal stem cells from adipose tissue (for example) for a variety of chronic degenerative conditions. One key element is the need to collect, harvest and return the cells in a timely manner (ideally within one to two hours). This approach is not a viable option when significant cell expansion, which may take many days or weeks, is required to provide sufficient cells for a dose.
NPP solutions have seemed a long way off, particularly if our reference point is a complex process such as CAR-T therapy. The ideal approach would be a single patient appointment where the entire process (cell collection, cell selection, modification and administration) is completed. The critical step of product release testing is a current challenge. Only when release testing can be incorporated and performed very rapidly (<1Hr), would an NPP approach be possible. Technology advancement in sensors and real time monitoring offer hope that this is not too far away.
Gene Therapy is one area where NPP seems plausible provided no expansion step is needed. Success will require that cells are collected, the required sub-set selected, modified and rapidly (<6Hrs) returned to the patient and produce the desired clinical effect.
The full potential of regenerative medicine is just beginning to be realized and CAR-T therapies are leading the way. RM medical devices have by in large taken a back seat as significant issues around complex cell manufacturing processes are addressed. As current technical challenges are resolved, NPP approaches will become viable solutions, the tissue-engineering sector will gain traction, and medical devices will become a critical element to successfully providing these therapies.
Brian Hanrahan is a Biotech Program Director. A customer-focused leader and program manager, Brian has more than 20 years of experience delivering successful product development in the biomedical and cell therapy industries.
Images: StarFish Medical
