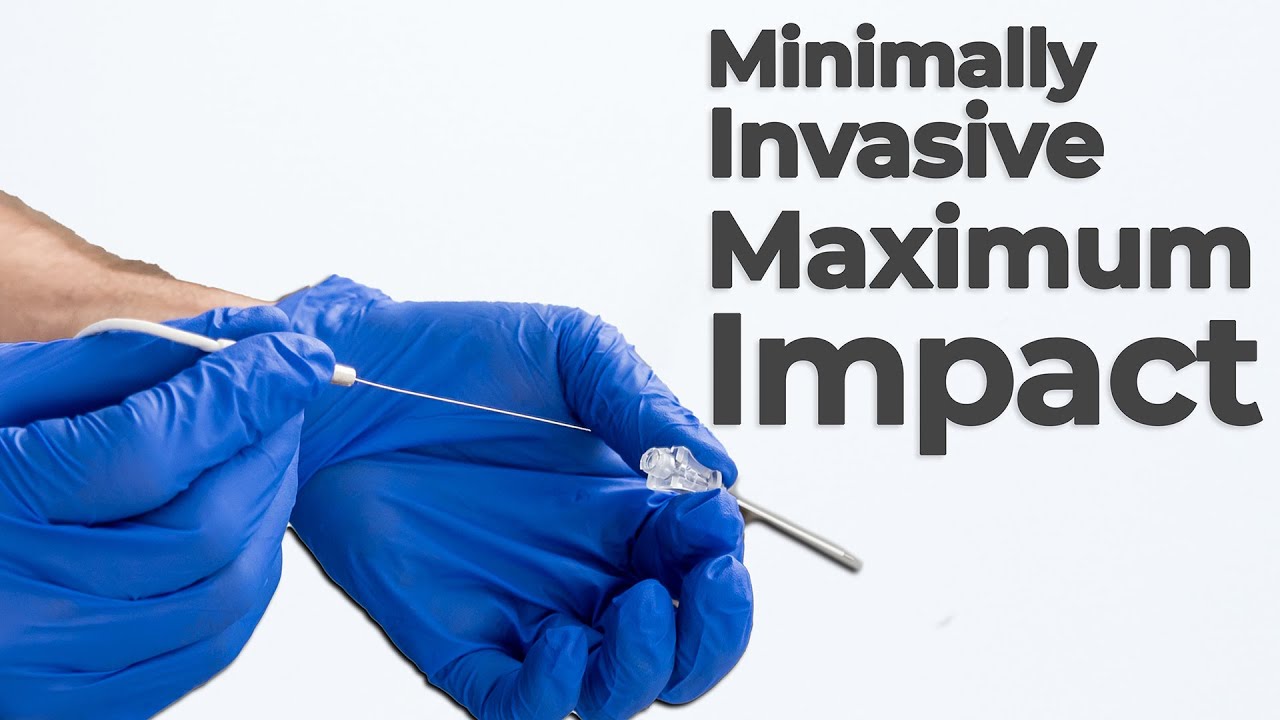
Bio Break: The Power of Ablation
In this episode of Bio Break, Nick and Joris dive into the rapidly growing field of minimally invasive medical technologies, focusing on a powerful technique that’s reshaping patient outcomes: ablation.
Joris explains that where open surgery was once the norm—think long hospital stays and extensive recovery—today’s patients can often benefit from procedures that require only small incisions and short hospital visits. At the core of many of these advancements is ablation technology.
So, what exactly is ablation? It’s the targeted removal of tissue from the body, usually done by a healthcare professional using minimally invasive tools. There are several types (chemical ablation, electrical ablation, and thermal ablation) each suited to different conditions and tissues. But regardless of the method, the goal is the same: safely and precisely eliminate unwanted or problematic tissue while minimizing disruption to the body.
Nick and Joris explore some of the most compelling use cases. From treating small tumors to managing chronic pain and even correcting cardiac arrhythmias, ablation is proving to be a versatile solution. One standout application is pain management, where ablation can target specific nerves responsible for chronic pain, such as persistent back issues. In cardiology, it’s commonly used to address irregular heart rhythms by disrupting malfunctioning electrical pathways.
And while the procedure offers numerous benefits, shorter recovery times, reduced risk of complications, and improved patient comfort, there are also important considerations. Since the procedure doesn’t involve fully opening the body, visibility is limited. That’s why sophisticated medical imaging is critical to its success. As a result, ablation is typically performed in advanced hospital settings with access to the right tools and expertise.
The Power of Ablation
Related Resources

Electrocardiography (ECG) remains the gold standard for non-invasive cardiac assessment, providing a vital window into the heart’s electrical health. By recording electrical impulses through surface electrodes, clinicians can identify life-critical conditions such as arrhythmias, myocardial infarctions, and conduction abnormalities.
Wearable medical and wellness devices are increasingly commonplace in the healthcare field. For example, devices such as smartwatches incorporate sensors to provide continuous health tracking data to their users.

Nick and Nigel explore how much bacteria can exist on devices and why it matters. They explain that bacteria are everywhere.