Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Company values that StarFish employees use most to develop better medical devices include looking deeper, cutting to the chase and getting better.
-
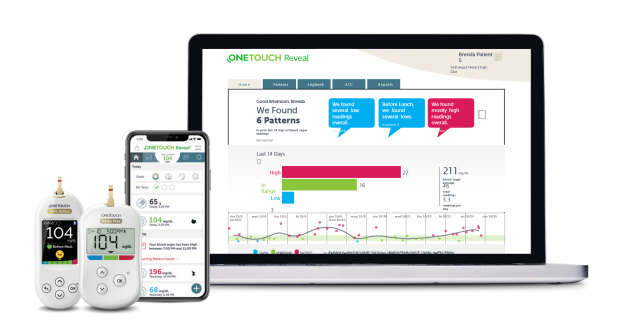
Medtech trends including AI, UX Design, Device Connectivity, and Sterilization Protocols that might impact your medical device project.
-
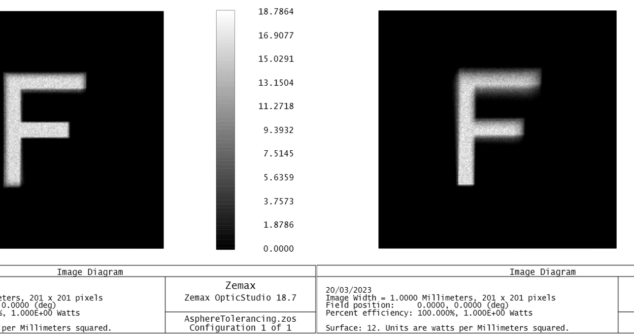
Overview of tolerancing simulation for medical device optical systems in Zemax OpticStudio’s sequential mode.
-

Top medical device project management lessons learned from more than a quarter century of product development experience.
-

Considerations for designing UDI labels for medical devices include ANSI/ISO Parameter Values, label verification and durability.
-

Guide to run a lean and agile medical device product development programs using with four considerations and key milestones.
-
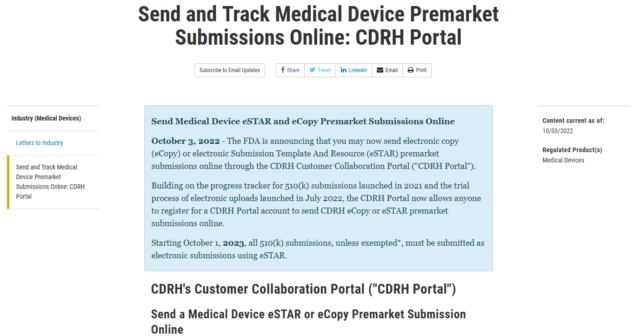
Overview of eSTAR, a joint Health Canada and FDA program streamlining medical device submissions with info on how to apply for the program.
-

Three concepts to improve teaming in medical device project management group are Handshakes, 5 Stages, and 6 Hats.
-

Five tips to develop robust medical device commercialization strategies that engage a cross-functional team from engineering, production planning and supply chain.