Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
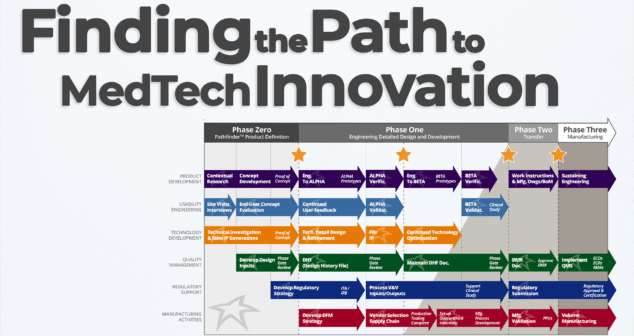
Nick and Joris explore one of the most dynamic early-phase services at StarFish Medical: the Pathfinder Program. If you're a medtech innovator with a promising concept or prototype, Pathfinder helps you identify the right path forward—before you invest millions in development.
-

Nick and Joris explore the wide world of ablation technologies—unpacking how each approach works and what it’s best suited for.
-
Nick Allan and Joris van der Heijden dive into one of the most impactful trends in modern medtech: minimally invasive surgery. Ablation technology plays a crucial role as hospitals and healthcare providers aim to reduce patient recovery times and overall system strain.
-
Optics Physicist and Engineer share approaches to performing pre-screen Ophthalmic Instrument Safety Assessment testing in-house.
-

If you’ve ever been to the hospital, you’ll know that one of the first things hospital staff do is attach “that finger clip device” to your finger. “That device” is called a Pulse Oximeter, and it provides information on pulse rate and blood oxygenation.
-
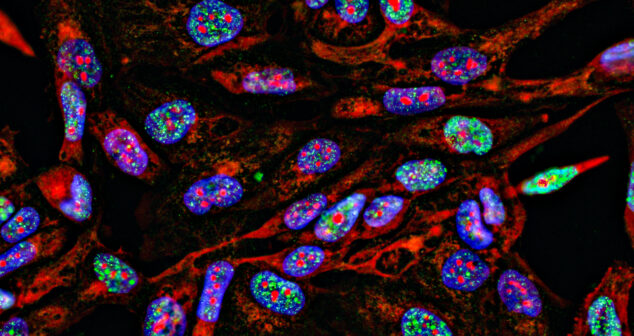
Fluorescence Imaging in Medical Devices outlines medical applications and examples of devices that use fluorescence for imaging.
-
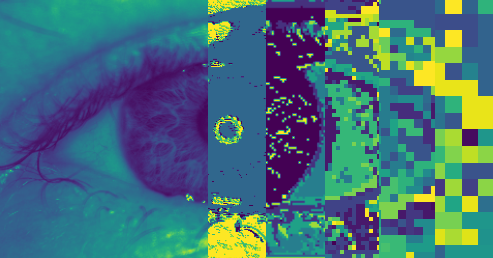
Computer Vision for Medical Devices is constantly evolving and incorporating new techniques and technologies as they emerge.
-
In this episode of Bio Break, Nick and Joris dive into the rapidly growing field of minimally invasive medical technologies, focusing on…
-

Being able to control the release rate of a target molecule is a valuable tool for engineering tissues and therapeutic delivery of regenerative medicine applications.