Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Questions and resources to help you find the right medical device development partner for your unique needs and requirement.
-
2020 Medical device development videos include COVID implications for research and POC devices, optimizing founder value, manufacturing for NPI, IVD insights.
-
2020 “most read” Top 10 medical device development blogs from StarFish Medical employee experts and development professionals.
-
COVID-19 Ventilator Design team discuss their top insights and lessons learned from the time constrained, critical ventilator project.
-

How FDA Breakthrough Devices Program provides advantages for novel devices and can significantly reduce a product’s time to market.
-

Overview of COVID-19 testing authorizations and developments of the COVID-19 pandemic in the United States and Canada.
-
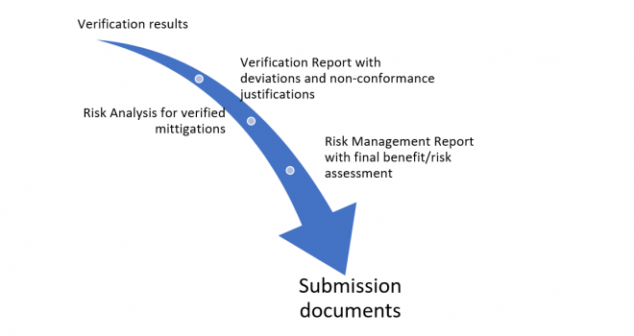
Author describes her experience submitting a medical device application (ventilator) under the Health Canada Interim Order.
-

Summary of expert advice from the Medical Device Coverage & Reimbursement Conference to help guide medical device reimbursement journey.
-

Knowing which medical device standards apply to your device is an essential part of successfully bring a medical device to market.