Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Cleanroom best practices are crucial to maintain a contamination-free environment, especially in industries like pharmaceuticals, semiconductors, biotechnology, healthcare, and medical devices. Here are some essential guidelines for ensuring your cleanroom is indeed clean.
-

Medical device design transfer is a critical phase in the development process, marking the transitory phase from the design and development stage to manufacturing. This phase ensures that the medical device continues to meet its safety, effectiveness, and regulatory compliance once production begins. It is the final phase in the development process and sometimes overlooks or underestimates the amount of time and effort required to ensure that final manufactured devices meet requirements.
-

Systems-thinking must always be present in medical device development. Systems Engineers (SEs) live where complex development needs managing. In a very small project team people can communicate continuously and tightly enough that everybody understands where they're headed and what's going on.
-

In this special New Year’s episode of Bio Break, Joris van der Heijden and Nick Allan reflect on resolutions, persistence, and a 20-year journey to establish a new standard for biofilm testing in medical devices. Nick shares the story of his two-decade-long mission to develop a standardized test method for growing and monitoring biofilm on medical device surfaces.
-

In this episode of Bio Break, Joris and Nick explore the fascinating ways microbes and nature inspire medical innovations, showing how these tiny organisms play an outsized role in advancing healthcare and biotechnology. From lifesaving antibiotics like penicillin to revolutionary technologies like CRISPR-Cas9, they dive deep into the surprising and transformative contributions of microbes to medicine, shedding light on their critical role in shaping modern science and improving patient outcomes.
-
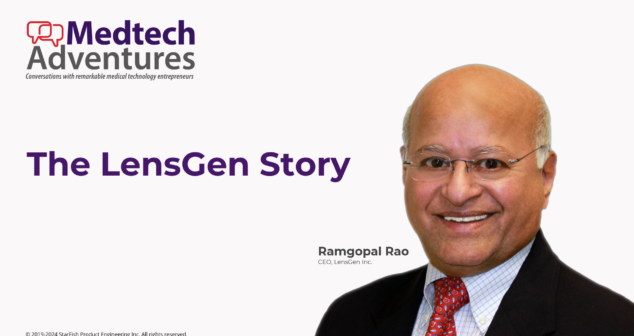
Explore the inspiring story of Ramgopal Rao, a trailblazer in the ophthalmic medtech industry. From humble beginnings in India during the era of partition to building groundbreaking innovations in the U.S., Ramgopal’s journey epitomizes resilience, vision, and entrepreneurial success.
-
2024 Medtech Entrepreneur Webinars feature both standard expertise webinars and livecast recordings from Playbook events in Vancouver, Canada and Newport Beach, California.
-

2024 Top Medical Device Blogs written in 2024 and the 10 most read evergreen blogs during 2024 include three new authors, two group blogs and five articles from blogging veterans.
-

2024 Top Medical Device Videos features a round up of most viewed medical device videos from StarFish Medical includes a mix of new videos released in 2024 along with evergreen videos sharing medical device expertise.