Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

9 medical device commercialization resolutions recommended by our team of engineers, designers, QA/RA, and manufacturing professionals.
-
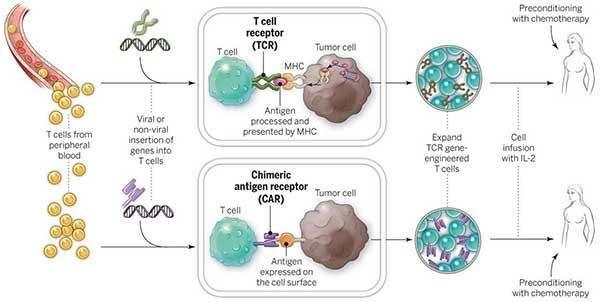
Chimeric antigen receptor T-cell (CAR-T) therapy is a rising shining star in Regenerative Medicine. Medical devices will be involved.
-

Advice to help companies determine when to start thinking about setting up a Startup Quality Management System.
-
Watch the most viewed StarFish Medical videos of 2018 to learn about medical device design, development, regulations, and manufacture.
-

New medtech blogs and new authors join our 2018 “most read” list. Regulatory was the most popular topic, followed by Electrical Engineering.
-

The Bleeding Edge explains how 510K cleared medical devices are clinically proven to improve patient outcomes and save lives.
-

The book Bad Blood eloquently demonstrates the importance of properly developing medical devices under ISO 13485.
-

6 benefits mean the FDA program for breakthrough medical devices may be your best regulatory option for novel medical devices.
-

FDA's Early Feasibility Studies Program (EFS) is a great option in early stage development looking to advance your program by gathering data.