Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Biotech in medical device companies: 3 compelling reasons to pursue a Bio Services career in medical devices.
-

Medtech Job hunters during and after COVID should pay attention to these six changes and adjust their search techniques and tools accordingly.
-
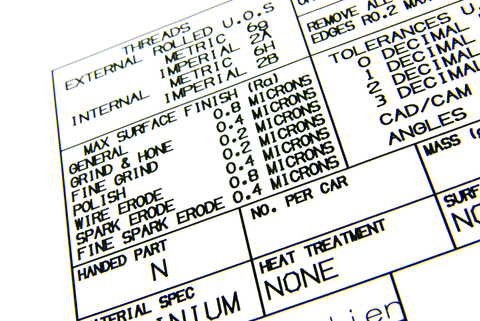
Tolerancing is the consideration of process accuracy (and, to some extent, precision) when designing a device or component.
-

Worst mistake when developing a medical device compilation from engineering, design, quality and regulatory experts.
-

Ingress protection for home healthcare medical devices covers the 60601-1-11 collateral standard and what developers should know.
-
COVID-19 Ventilator Design team discuss their top insights and lessons learned from the time constrained, critical ventilator project.
-

Formative Evaluations and Tests details the differences between these two methods, and the appropriate circumstances for their application.
-

COVID-19 friendly brainstorming meetings identifies 5 benefits where virtual brainstorm results outperform in-person brainstorming results.
-

How FDA Breakthrough Devices Program provides advantages for novel devices and can significantly reduce a product’s time to market.