Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

ISO 10993-1 and Biocompatibility impact on Medical Device Plans. Financial impact of additional Medical Device testing will have a cost.
-
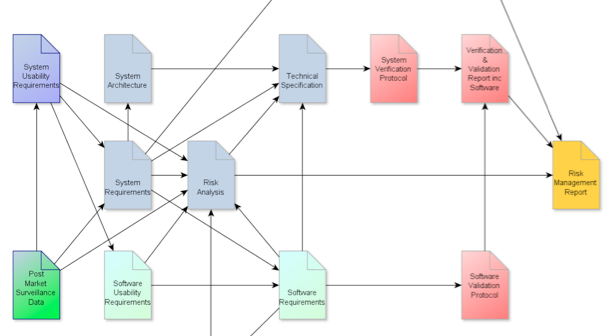
Seven tips for a smooth and successful outcome.to IEC 60601 Edition 3 submissions and electrical safety testing
-

Preparation for our medical device development and contract manufacturer On-Site inspection helped our client earn a successful Factory Inspection.
-

When designing medical devices, use trustworthy data sources! Reading the manufacturer’s spec sheets and errata is not sufficient for problem-free design.
-

Updated FDA Recognized Standards for use in premarket reviews of medical devices with declaration of conformity to consensus standards.
-

Medical Device Internal Auditing is vital in device development, but the results of an internal audit are not always easy to hear.
-

Enjoy a smooth product development ride by apply an extreme risk management approach for device, manufacturing and project.
-

Proposed EU Medical Device Regulations (2012) replace current Directives with two Regulations; one for medical devices and the other for IVDD
-

Article with update links on 2012 US Medical Device Tax in the US and blogs with controversial views of the tax impact on the industry.