Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Overview of COVID-19 testing authorizations and developments of the COVID-19 pandemic in the United States and Canada.
-
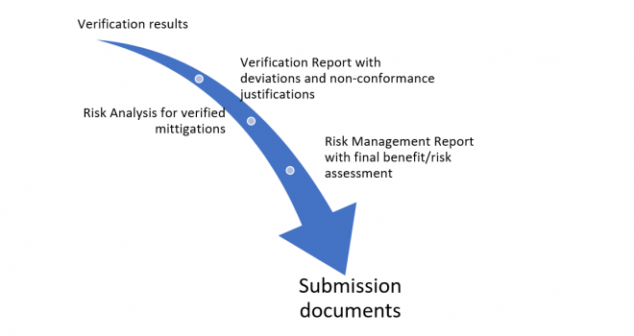
Author describes her experience submitting a medical device application (ventilator) under the Health Canada Interim Order.
-

Summary of expert advice from the Medical Device Coverage & Reimbursement Conference to help guide medical device reimbursement journey.
-

Knowing which medical device standards apply to your device is an essential part of successfully bring a medical device to market.
-

Inspiration for work from engineers, regulatory, quality, design, and program management creative professionals in medical device development.
-

Health Canada improvements: Getting new devices to market, strengthen monitoring and follow-up and provide more information to Canadians.
-

FDA provides a roadmap to follow for premarket submission in their guidance on Content and Format of Non-Clinical Bench Performance Testing.
-

Most viewed StarFish medtech videos in 2019: StarFish Medical videos share insights and tips on medical device design, development and manufacture.
-

Top 2019 Medical device commercialization videos and lessons from StarFIsh Medical development and commercialization experts. Based on actual medical device cases.