Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
Three common elements can be observed in biomedical product development projects that are doomed to failure. They are the Three “P’s” – Platform, Point of Care, and Program Manager.
-
Medical device post-market surveillance (PMS) is important to identify and address potential safety issues and improving device performance. Article covers regulatory landscape, challenges, innovations and collaborative efforts.
-
Understanding similarities and differences between Medical Device 510(k) and CE Marking regulatory pathways helps harmonize overall regulatory strategy.
-

Environmental, Social and Governance (ESG) Medical Device Impact tips to ensure that your products fulfill compliance obligations. Major buyers request ESG reporting from suppliers as new and revised regulations require supply chain attention.
-

Overview of 2024 Regulatory trends include QMSR, LDTs, FDA guidances, and timelines in the EU for MDR and IVDR.
-

FDA Real-World Evidence Draft Guidance analysis identifies five key points to help understand the potential impact of the draft guidance.
-
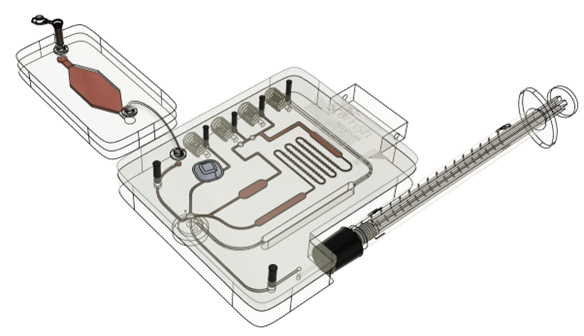
How a ready-made modular proof of concept system for rapid prototyping Point of Care Diagnostics can expedite and de-risk product development.
-
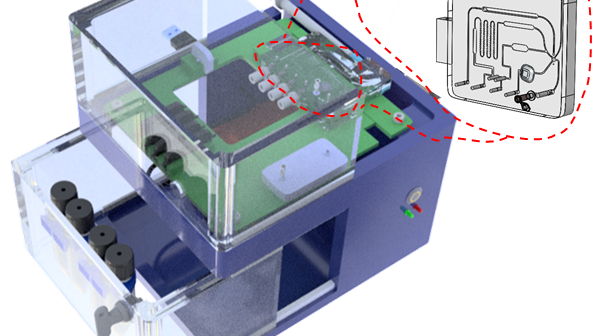
The modular platform for transferring immunoassays acts as an intermediate bridge and is a significant step towards streamlining the product development process for diagnostics.
-
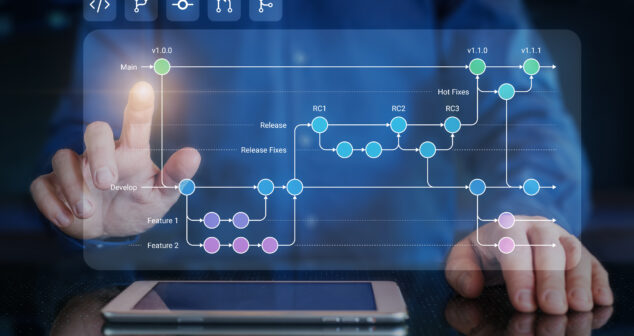
Medical device software is very diverse. In all this variety, one technology is ubiquitous: Git! Three eye-openers which help developers use Git better.