Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

9 ways a Medical Device development project project manager (PM) is critical for medical device development, and manufacture.
-

Ways to hone focus, and help ensure early requirements definitions are well formed to solve the right problems in medical device projects.
-

Sometimes the best way to secure regulatory approval requires the project manager to build a different box.
-

Top 10 2015 StarFish Medical videos help connect viewers with employees they may only know from reading or phone calls.
-

Top 2015 medical device blogs written by StarFish Medical expert employees sharing their knowledge, tips, and tools.
-

Expedited Access Pathway (EAP) is promising for timely approval and access to devices for life-threatening or irreversibly debilitating conditions.
-

Recent report on policies and initiatives claims the FDA device arm (CDRH) medical device approval process is much faster than five years ago.
-
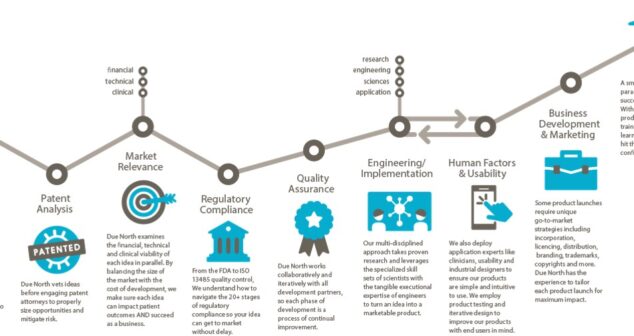
Sketch to Launch commercialization process for medical devices guides translational services to the medical industry.
-

Advice, implications and examples for best time to implement a medical device Quality Management System in US, Canada and Europe.