Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
Commonalities and points of potential confusion of the “big 4” incoherent-light hazard classification and standards for medical devices.
-

Top medical device project management lessons learned from more than a quarter century of product development experience.
-

Revolutionizing Wound Care examines smart bandages with wearable sensors using microfluidics for wound care.
-

Five tips to develop robust medical device commercialization strategies that engage a cross-functional team from engineering, production planning and supply chain.
-

The risk management approach
-
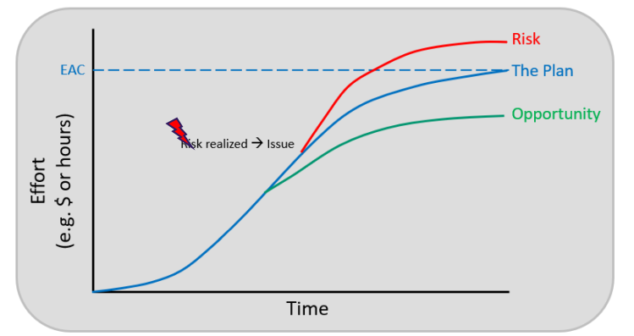
Proven approach to medical device development risk management using an every day concept using plan, risks, opportunities, and actions.
-
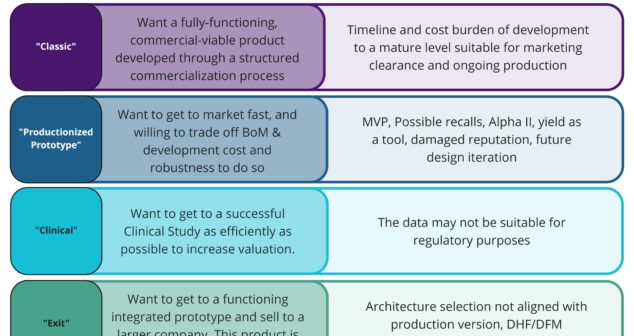
Six common medical device product development destinations and overview of a High Level Program Planning (HLPP) approach.
-

Learn about the FDA's 2023 guidance on premarket submissions for device software functions and its impact on the medical device industry.
-

The FDA released a new Cybersecurity draft guidance on April 2022. It is intended to replace the current final guidance from 2014 which is well overdue an update. The draft guidance significantly expands requirements for cybersecurity activities and documentation for medical devices. The intention is to align medical device development with current best practices from other industries. This white paper reviews these new requirements and considers their impact on medical device developments.