Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
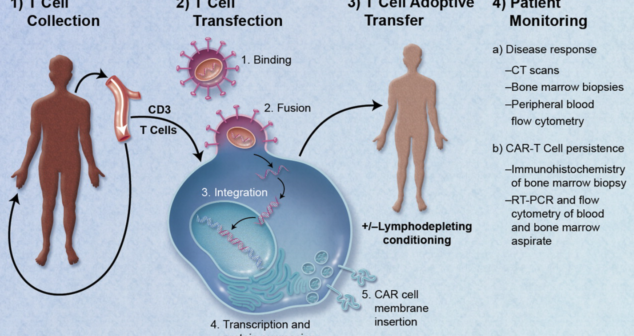
Regenerative Medicine (RM) therapeutic medical devices transform healthcare. The role of Medical Devices in the RM industry is changing.
-
Watch the most viewed StarFish Medical videos of 2018 to learn about medical device design, development, regulations, and manufacture.
-

New medtech blogs and new authors join our 2018 “most read” list. Regulatory was the most popular topic, followed by Electrical Engineering.
-

attribute that should be a high priority in any launch plan. Manufacturer flexibility could end up being one of the most important factors to an efficient and cost effective product launch that fits your business objectives.
-
NPI Medical Device Manufacturing Common Pitfalls and how to solve them by increasing volume in a small and controlled way.
-

Reduce hidden costs by increasing Supply Chain visibility to answer the common supply chain question: “How can we reduce the project cost?"
-

Lean small scale manufacturing is highly effective for efficiencies, health and employee engagement and increased productivity.
-

NPI team's top tips for production readiness based on their collective experiences transferring medical devices from design to production.
-

PFMEA prevents causes rather than detecting them (or the effects of failures), and should be incorporated into medical device design.