Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Highlights of July and August 2014 medical device FDA guidance documents that reduce regulatory burden without compromising patient safety.
-

Tips, considerations, and techniques to increase IVD Regulatory Success that is essential for many Medical Devices.
-

Will consumer electronics engulf the medical device development community, making rigorous testing and risk mitigation processes obsolete?
-

MDSAP enables Medical Device companies to save in Registrar’s fees, eliminate duplication of inspections and gain faster access to markets.
-

Results of StarFish client satisfaction survey with a large group that included customers and prospects using one word descriptions.
-

Regulatory affairs expert explains how to write a best practice ISO 9000 Medical Device Quality Manual using 3 simple rules.
-
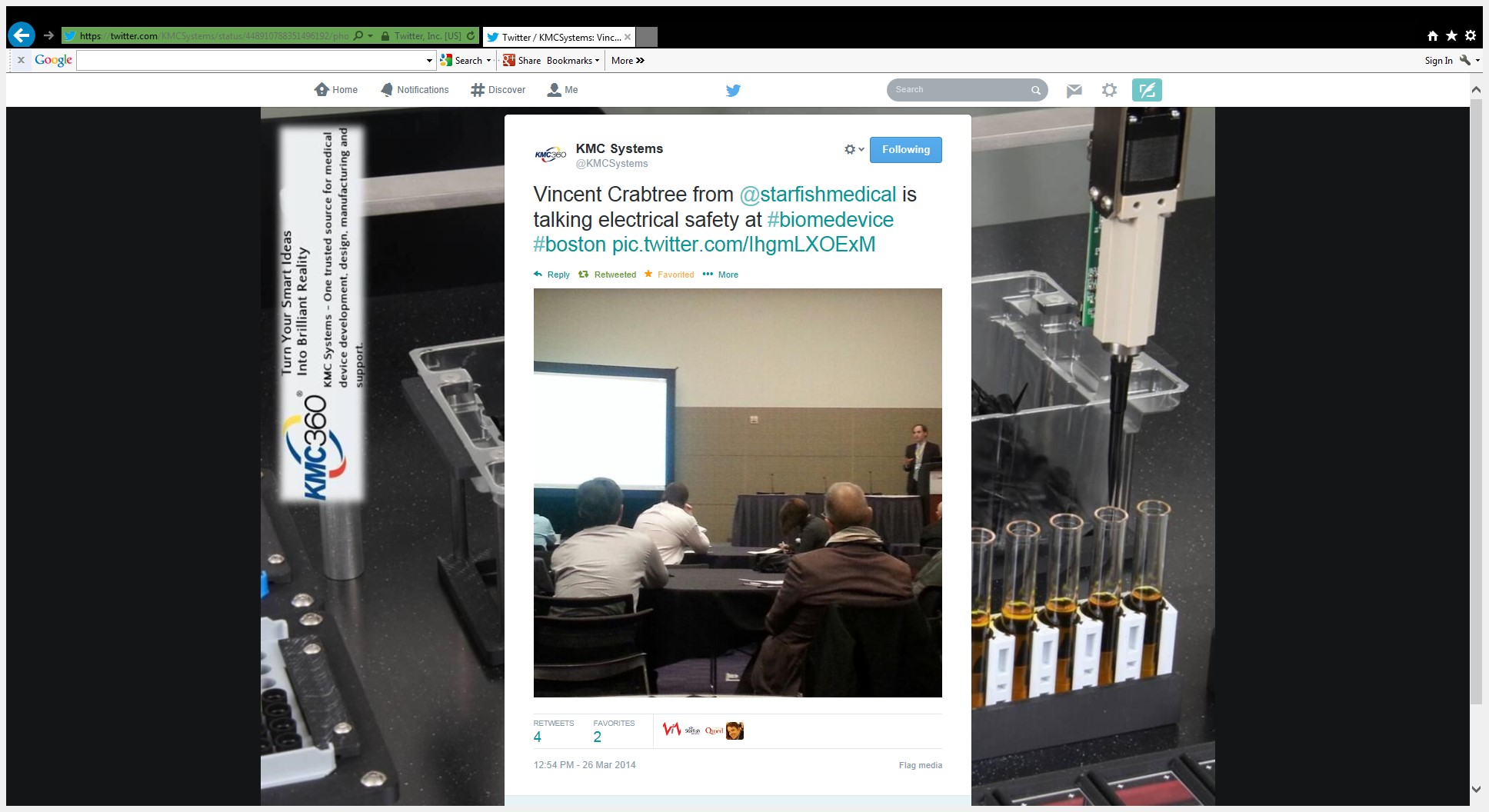
Highlights from Day 1 of Learning Lab event at Boston BIOMEDevice on electro-medical device basic safety and achieving compliance with 60601.
-
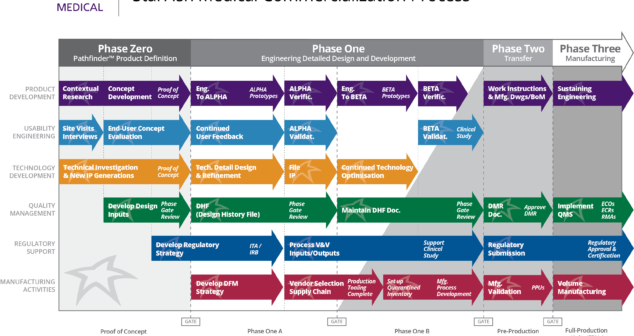
Medical Device Design and Development Process Improvement for Annual Plan: limit improvement efforts, measure them regularly, succeed.
-

The best way to avoid waste is to consider medical device regulatory requirements at a project outset.