Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
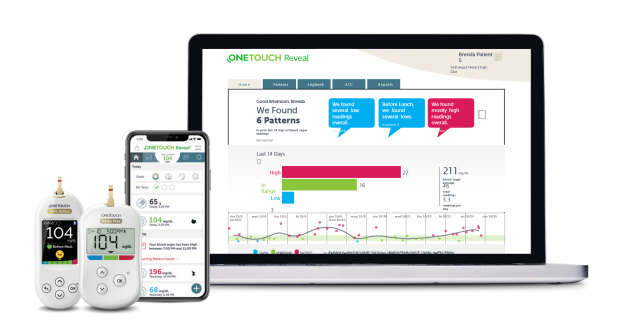
Medtech trends including AI, UX Design, Device Connectivity, and Sterilization Protocols that might impact your medical device project.
-
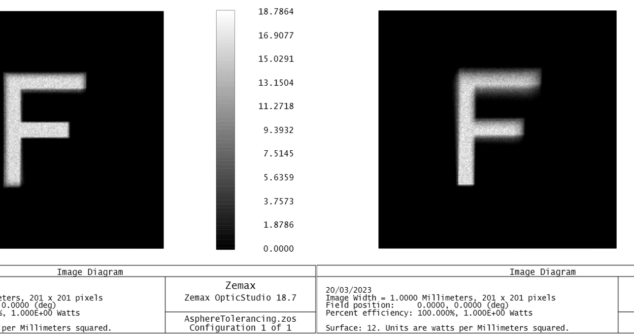
Overview of tolerancing simulation for medical device optical systems in Zemax OpticStudio’s sequential mode.
-
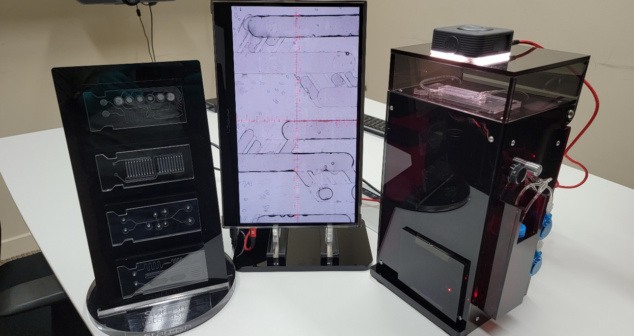
Building an affordable customized Microfluidics Microscope set-up to view droplets as they are being generated.
-
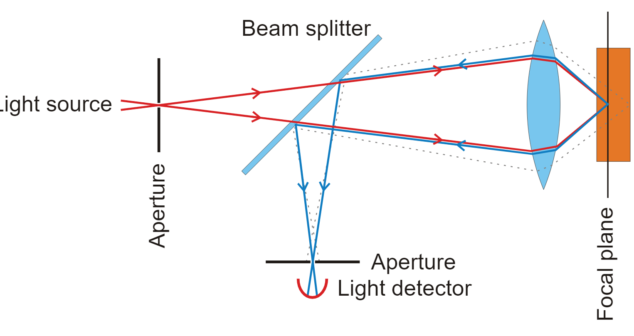
Optical Imaging Biomedical Devices integrate optical imaging with conventional medical imaging devices to enhance biomedical imaging.
-
Commonalities and points of potential confusion of the “big 4” incoherent-light hazard classification and standards for medical devices.
-

Top medical device project management lessons learned from more than a quarter century of product development experience.
-

Interference Testing, Strategy, Detection Case Study examines POC medical devices assay verification guideline CLSI EP07-A2.
-

How an Autonomous Clinical Chemistry Assay diagnostics design with kinetic read out helps mitigate interference challenges.
-

Five tips to develop robust medical device commercialization strategies that engage a cross-functional team from engineering, production planning and supply chain.