Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Proper research and communication with authorities in each country of interest will make Medical Device International Regulations smoother to navigate.
-

Dr. Joe Burkett, an emergency medicine physician shares his Medtech entrepreneur journey as Founder & CEO of Vaid Medical Devices.
-
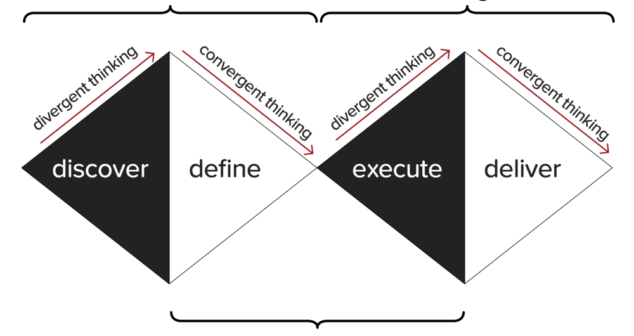
Kepner-Tregoe’s original Decision Matrix overview with tweaks to make it broader and more robust.
-

Five things project managers can do to achieve great results and empower team members to perform at their best.
-

Managing evolving requirements is a core competency for medical device technical specifications and understanding health care end users.
-

11 medical device development mistakes that surfaced frequently in a variety of products. Avoid them to reduce cost and time to market.
-
Assay industry trends overview includes infectious diseases diagnostics industry continuing to expand into traditional diagnostic areas.
-
GDPR is designed to enhance the privacy and security of data subjects. Medical devices and GDPR cross paths in many areas.
-

Our employees' medtech resolutions include great ideas. What are your improvement plans? We'd love to hear and share them.