Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Comparison of the biggest unknown between IEC 62366:2007 and IEC 62366 -1:2015– Fig. 5.10 User Interface of Unknown Provenance (UOUP).
-

11 Lessons learned from medical device project implementations cover a range of topics from regulatory and quality to development.
-
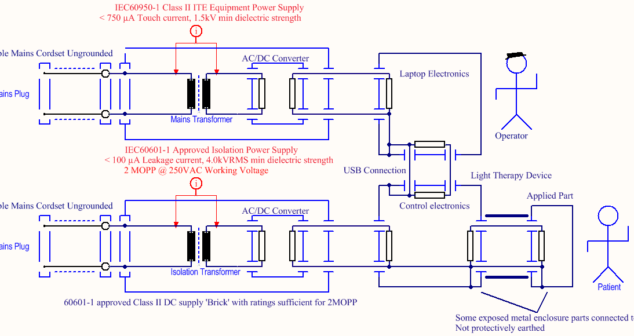
Creating an electrical insulation diagram early on helps to choose the right parts and is required by IEC 60601-01.
-

Overview of FDA Regulation of E-cigarettes Deeming Tobacco Products to be subject to the Federal Food, Drug and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act.
-

Digital Health communication technology cost and deployability for medical devices features a discussion of StarFish engineers
-

Electrical Engineer's overview of IEC 60601-1 covering Terminology & Definitions through Annexes. Part 2 of 2 blogs on the topic.
-

EE's overview of IEC 60601-1 Scope and Normative References, one of the more important standards that apply during medical device development.
-
13 ways a PMO (Project Management Officer) manages their responsibility for all of the actions a company does in project management.
-

What’s the best way to motivate medical device teams? What used to work may not apply any more.