Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
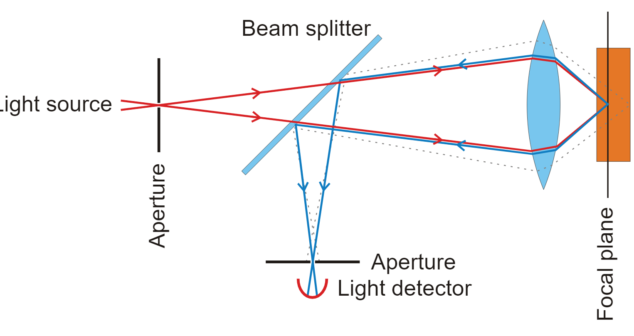
Optical Imaging Biomedical Devices integrate optical imaging with conventional medical imaging devices to enhance biomedical imaging.
-

Review of April 2023 FDA draft guidance on PCCP Recommendations for AI/ML-Enabled Device Software Functions.
-
Commonalities and points of potential confusion of the “big 4” incoherent-light hazard classification and standards for medical devices.
-

Top medical device project management lessons learned from more than a quarter century of product development experience.
-

Considerations for designing UDI labels for medical devices include ANSI/ISO Parameter Values, label verification and durability.
-

Overview of computational modeling and simulation (CMS) and its impact on future medical device development.
-

Guide to run a lean and agile medical device product development programs using with four considerations and key milestones.
-

Interference Testing, Strategy, Detection Case Study examines POC medical devices assay verification guideline CLSI EP07-A2.
-

How an Autonomous Clinical Chemistry Assay diagnostics design with kinetic read out helps mitigate interference challenges.