Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Top 10 StarFish Medical blogs of 2017 feature a mix of regulatory, engineering, and practical information and advice for all levels of expertise.
-

Enjoy our 2017 Readers' Choice Blogs and StarFish Medical articles, images, events and videos from our monthly newsletter.
-

StarFish Medical videos help viewers learn medical device design, development and manufacture insights and tips from the people at StarFish.
-

RoHS and REACH are two regulations that requires compliance if a company intends to sell in the European Union.
-

Establishing supply chain controls to ensure reliable suppliers is critical to satisfy regulatory requirements for your device.
-

Top 3 root causes of design transfer problems are identified by the author with suggestions for corrective actions to mitigate the problems.
-

Applying Process Failure Modes and Effects Analysis (PFMEA) efficiently to Medtech manufacturing process will greatly improve effectiveness.
-

A world class medtech supply chain is made on a foundation of controls, knowledge and planning. Expert tips for optimization.
-
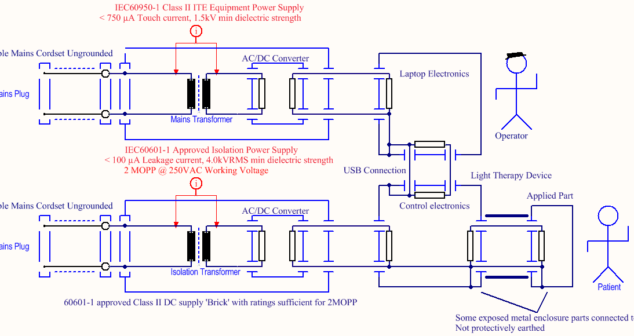
Creating an electrical insulation diagram early on helps to choose the right parts and is required by IEC 60601-01.