Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Tips and examples for Product Definition (Phase Zero) of medical device commercialization process.
-

Analysis of FDA draft guidance "Decentralized Clinical Trials for Drugs, Biological Products, and Devices."
-
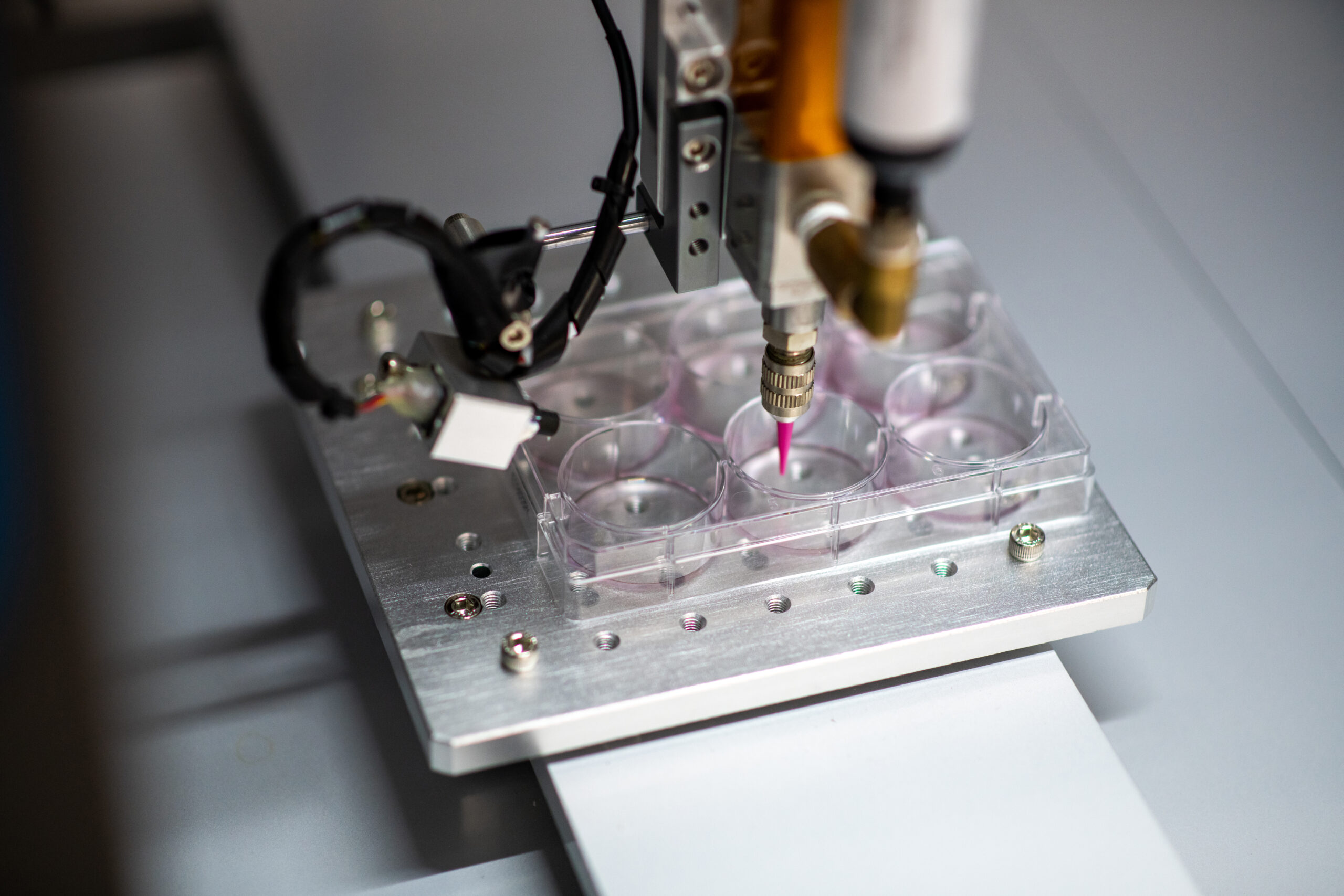
Bioprocessing (biologics manufacturing) 4.0 requires innovation in bioprocess equipment design, control and integration.
-

Systems engineering can help early-stage biotech companies overcome challenges associated with technology transfer.
-

Understanding lipid nanoparticles and microfluidics, the technology behind the unprecedented speed of vaccine production.
-

Company values that StarFish employees use most to develop better medical devices include looking deeper, cutting to the chase and getting better.
-

Zombie Microfluidic Cartridge early indicators to determine if a cartridge will function as designed or fail during the assay protocol run.
-
Medtech trends including AI, UX Design, Device Connectivity, and Sterilization Protocols that might impact your medical device project.
-
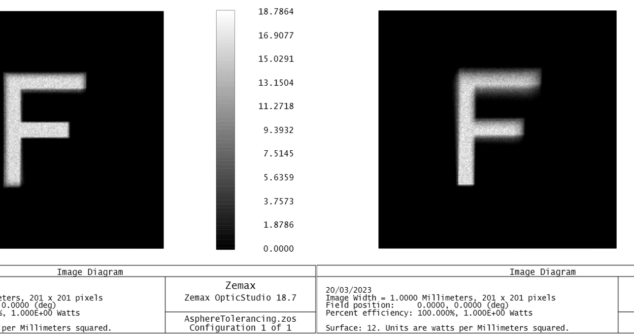
Overview of tolerancing simulation for medical device optical systems in Zemax OpticStudio’s sequential mode.