Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Most read StarFish Medical Device Development Blogs of 2014: regulatory information, analysis, testing, design, career path, and finances.
-
Staffing a Medical Device Start-Up: shortlist of job descriptions required to develop a complex, novel medical device.
-
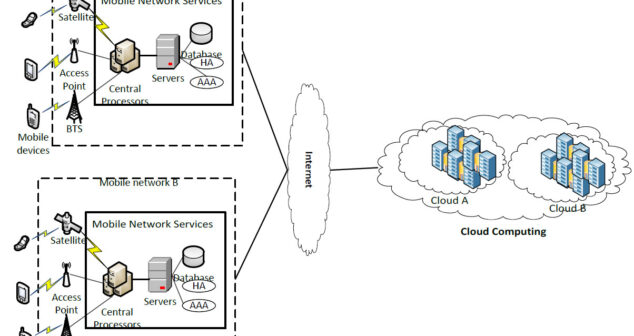
Privacy and cloud-based mobile apps integrated into mobile medical apps. Summary of privacy considerations.
-

Highlights of 510(k) and CE Marking similarities and differences. Explore the Quality Management System (QMS) and Documentation aspects.
-

Medical Device 510(k) and CE Marking similarities and differences to help companies effectively prioritize their regulatory efforts.
-

FDA MDDS and mobile medical applications guidances; what they mean & where they apply. Article identifies common sense approach by FDA.
-
Milestones and insights on taking a medical device idea from concept through to manufacture. Identifies a few false 'done's along the way.
-

4 tips to collect meaningful user data for emerging medical device technology and take an appropriate level of risk and reward.
-

Project Manager analysis of medical device development client-vendor perception gaps on cost, timeline, quality, and communication.