Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
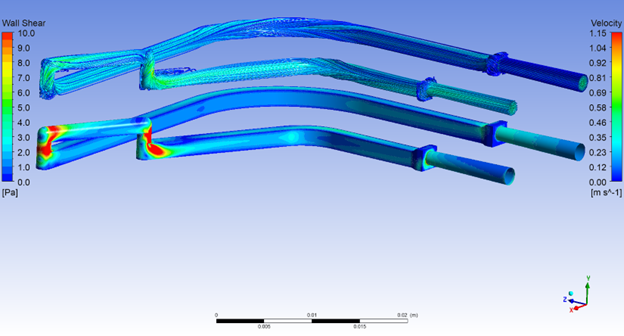
Drug Delivery Solutions for Cell and Gene Therapy (CGT) Challenges explores effective delivery devices and solutions for advanced CGT therapies and their unique challenges.
-
Filter Integrity Biopharmaceutical Manufacturing explores the relationship between microbial retention tests and integrity tests essential for validating the effectiveness of filters in critical applications.
-

Review of FDA Guidance on Using ISO 10993-1 integrating biocompatibility evaluation within the risk management process, as outlined in the FDA Guidance Document Use of International Standard ISO 10993-1, Biological Evaluation of Medical Devices Part 1: Evaluation and testing within a risk management process.
-

Integrating service design with user-centered design when developing medical devices can enhance device usability, efficiency, and outcomes.
-

7 Tips for conducting Voice of Customer medical device research in Low- and Middle- Income Countries (LMICs) is an integral component in user-centric design and development. It is increasingly an expectation of regulatory bodies.
-
The medical devices in the masthead above have vastly different purposes. One thing that’s common is they all have some combination of sensory aspects. Sensory design uses affordances (perception of design features intuitively suggesting a functional task) and semantics (conveying meaning and information about the product’s use and function through sensory cues). Both can be communicated through our five senses with the most common being visual, tactile and auditory.
-

Developing medical devices with software involves a wide-ranging set of unique challenges. Our software engineering team offer advice on offered advice on software in medical devices, FDA, Software as a Medical Device (SaMD), risks, cybersecurity, and Open Source software.
-

9 ways to use AI in medical device development from our experts include Connected Medical Devices, QA/RA, Developing (real time) Simulations, Expedite Design Process, Analysis and Research, Supplier Evaluations, Project Reviews, Brainstorming, and Administrative Help (Faster than Google).
-

Ensuring on-time medical device manufacturing builds are done in a timely manner requires coordinating across several teams including engineering, manufacturing, and quality. Visual Project Management (VPM) and Manufacturing Production Schedules (MPS) help improve manufacturing performance and meet build delivery deadlines.