Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Laser-Speckle-Contrast-Imaging is a non-invasive and inexpensive method of imaging flow of blood and other optically scattering media.
-

Supply Chain constraints in the age of COVID-19 are troubling. Medical device purchasers supply chain tips for delivering the goods.
-
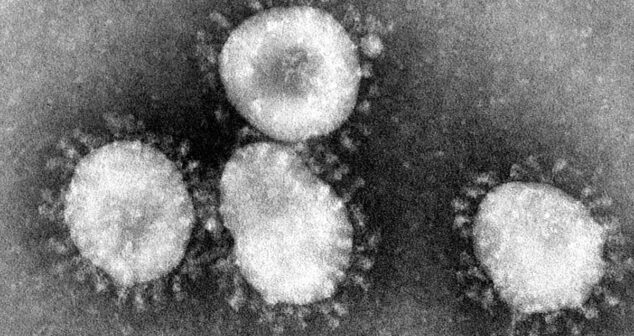
What is a coronavirus and why is a CoVID-19 Point of Care (POC) Diagnostic so important? Background and analysis of the vital role of POCs.
-

One response to possible COVID-19 medical device shortages is to try to hack DIY COVID-19 medical devices together. This is far more nuanced than it seems.
-

Creative Human Factors Processes for COVID-19 solutions to combat logistical hurdles for human factors research and evaluation activities.
-
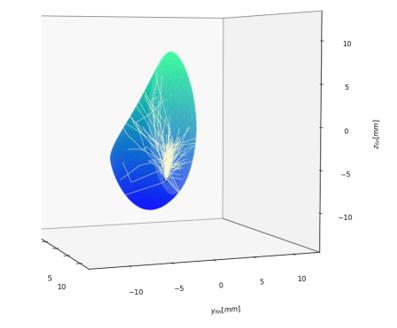
Monte Carlo Simulation for Biomedical Device Design: A common approach to simulate scattering is the Monte Carlo technique.
-
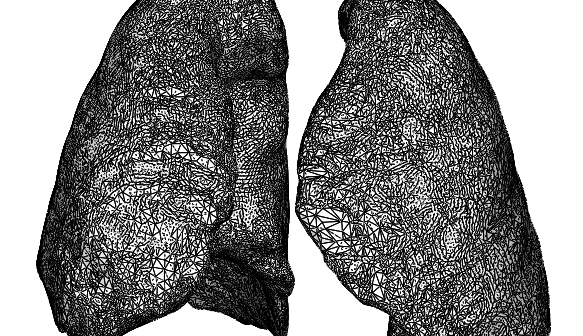
Create better functional models from anatomical scans with free tool for easy manipulation, simplification and cleaning up of mesh files.
-

Clean or Sterile? If a medical device does not need to be sterile, how clean does it need to be? In-depth review of terms and requirements.
-

Biological sciences medical device breakthroughs for this decade have a solid base to build upon. Here are our top five picks for the 2020's.