Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Designing Microfluidic Cartridges offers five critical considerations to increase success during microfluidic cartridge development.
-

Five examples of Microfluidic Machine Learning enabling microfluidic technologies to push into new areas and applications.
-
3D Printed Parts Streamline Development provides context for designers using 3D printed parts as a part of their market-ready medical devices.
-

Mechanical Engineering Team lead shares his response to students who ask him what the ideal medtech co-op looks like.
-

Medical Device Camera Sensor technical trade-offs to consider when incorporating a visible or near-infrared range camera sensor into a medical device.
-

Optical Safety for Ophthalmic Instruments covers safety limits and maximum exposure limits of wavelengths in the IR, UV and visible light spectrum.
-
Maximize medical device optical signals by keeping sensor and control signals correlated and other strategies are discussed in this article.
-
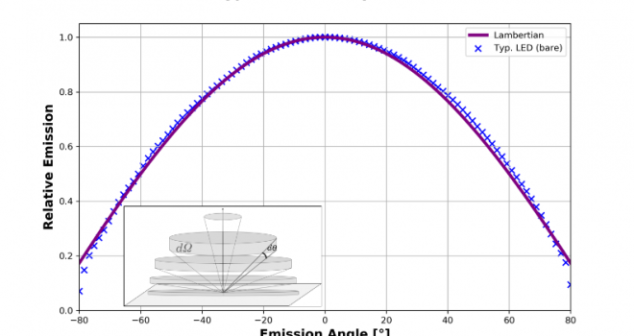
Coupling light from LEDs and light guides in Medical Devices overview and tips include putting the light-guide entrance face as close as possible to the LED output face.
-

Medical Device Design for the Developing World – why are there so many nonfunctional and broken medical devices in low-income regions?