Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Translating point of care assays into a simple, low cost and easy to use microfluidic cartridge is straightforward with these six steps.
-

Dr. Katherine Elvira speaks about her work developing lab-on-a-chip (microfluidic) technologies to investigate how drugs enter human cells.
-

Developing a “lab-on-a-chip” (LOC) solution brings unique challenges and benefits. 6 tips for developing a “lab-on-a-chip” (LOC) solution.
-
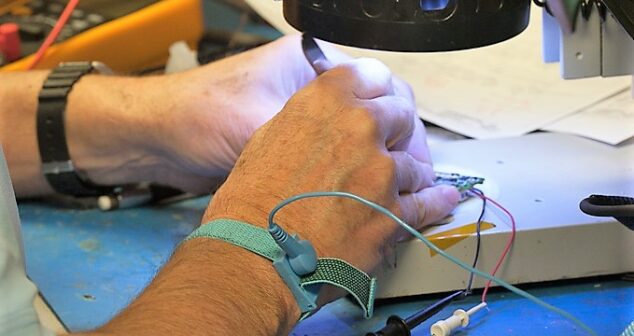
Pros and Cons of Designing a Custom PCB are debated in a group discussion of StarFish Medical electronic engineers.
-

The amount of total company funding to develop a Class II 510(k) cleared medical device is approximately $30 million. The development and engineering costs comprise approximately $2-5 million of this total. This estimate is built upon a meta-analysis of various references as well as our experiences in engaging with companies. Many factors influence these costs, including the need for clinical studies, regulatory pathway, and technology complexity. Refinement of these numbers requires a professional team to understand the technology, regulatory, and business opportunities.
-

How to get the most from your FDM 3D printer covers all of the key things to keep in mind when designing parts for FDM 3D printing.
-
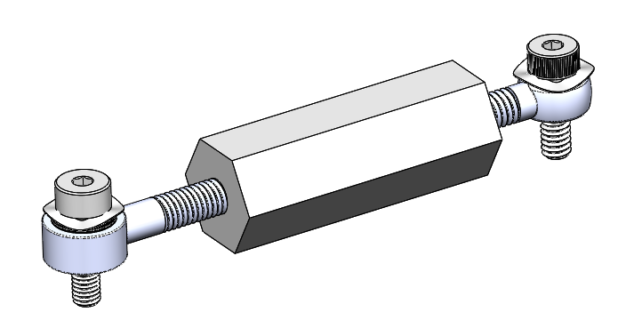
Design and construct a custom differential screw that is relatively inexpensive and easy to integrate with your system.
-

Most read StarFish medical device commercialization blogs of 2019 – Regulatory and electrical engineering articles tied, followed by biotech and supply chain.
-

Most viewed StarFish medtech videos in 2019: StarFish Medical videos share insights and tips on medical device design, development and manufacture.