Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

5 reasons Human Factors remain important in medtech despite technological advancements like Artificial Intelligence (AI).
-

Analysis of FDA draft guidance "Decentralized Clinical Trials for Drugs, Biological Products, and Devices."
-

Review of April 2023 FDA draft guidance on PCCP Recommendations for AI/ML-Enabled Device Software Functions.
-

Strategies to maximize outcomes for interactions during regulatory pre-submission meetings using 4 simple, but effective steps.
-

FDA cybersecurity requirements for medical devices which are considered “cyber devices”, including US government definition.
-
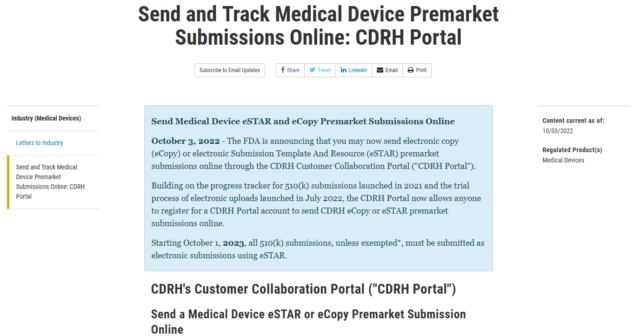
Overview of eSTAR, a joint Health Canada and FDA program streamlining medical device submissions with info on how to apply for the program.
-

Two new blog join eight from last year's top 10 on the list of most-read StarFish medical device blogs and articles for 2022.
-

Medical device product definition tips ranging from general guidelines to regulatory, reimbursement, and hazard management.
-

Engineering and QA/RA leaders share new technology, regulatory and supply chain developments that will impact future medical devices.