Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Medical device development experts share their biggest medical commercialization lessons learned over 20 years.
-

Installing metal inserts can ease the frustration of fastening 3D printed components. Overview of inserts and unique benefits.
-

Reduce medical device program risks with smart testing strategies – a systematic approach to device testing.
-
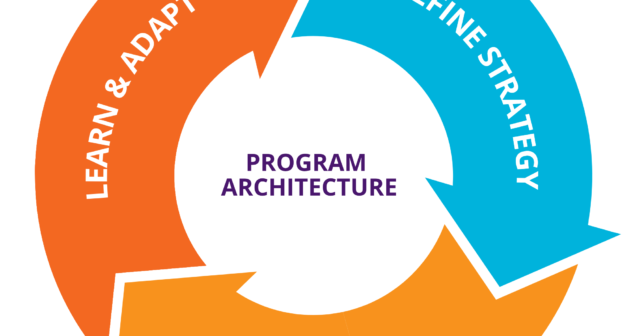
Medical device commercialization projects often need a Project or Program Manager to be the “Program Architect.” of Program Architecture.
-
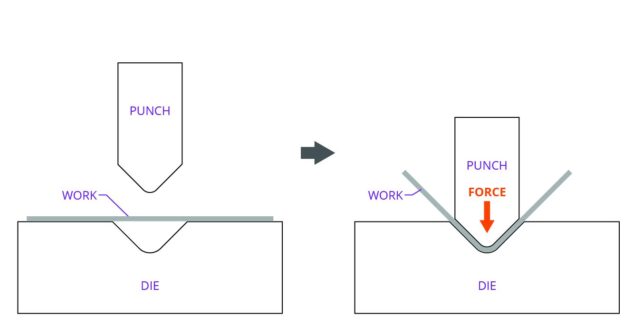
Sheet metal fabrication is an excellent method of making mechanical parts. This article explains how to avoid hole distortion in sheet metal parts.
-

Michael May, President and Chief Executive Officer of CCRM discusses the intersection of medical devices in Regenerative Medicine.
-
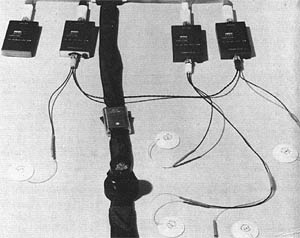
Apollo program engineers collected vital biomedical data from the astronauts using biomedical telemetry to monitor crew health.
-
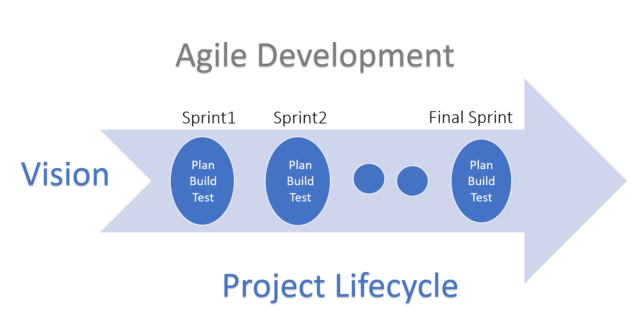
Agile methodology in Medical Devices Product Development methodology is supported by an FDA guidance and IEC standards.
-

Article offers 10 Ultra Low Power Embedded Design tips to meet growing demand for medical devices that do more with less power.