Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Top 2019 Medical device commercialization videos and lessons from StarFIsh Medical development and commercialization experts. Based on actual medical device cases.
-

Four questions one should address when considering Infectious Disease Diagnostics market potential by using public information.
-

5 simple tricks to reduce low volume supplier lead times and mitigate most medical device vendor supply time challenges.
-

Tips for tackling supply chain quality challenges in low volume medical device manufacturing from Kathy Young, Manufacturing Director, StarFish Medical.
-

Extract good value from your medical device supply chain by identifying potential cost savings and collaborative key supplier relationships.
-

Testing Medical Devices in a Biolab? Pre-planning is required for specialized tests, potentially dangerous microorganisms, tissues and biological fluids.
-

Tips and a quiz for picking and approving calibration equipment vendors for medical device verification and validation.
-

9 tips for 510(k) submission along with lessons learned from a recent successful experience submitting a medical device to the FDA.
-
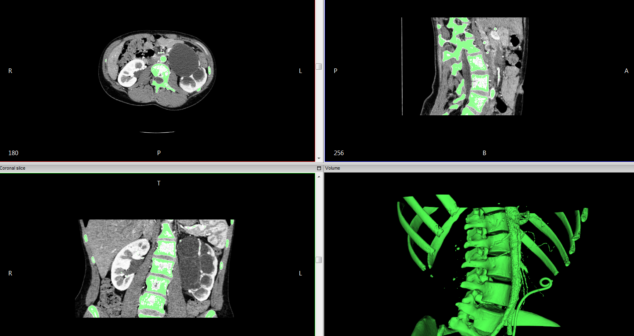
How to create accurate and functional 3D models from patient imaging data. Develop anatomically accurate prototypes for medical device design.