Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

11 medical device development mistakes that surfaced frequently in a variety of products. Avoid them to reduce cost and time to market.
-
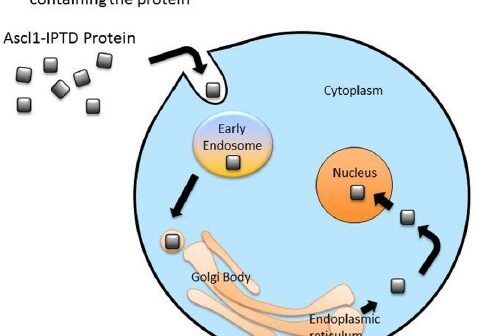
Cellular reprogramming is a strategy for a variety of medical applications where an adult cell is flipped from one type directly into another.
-

When selecting a method for developing an assay, whether it is for detecting bacteria or simulating fluid, the approach all depends on…
-
GDPR is designed to enhance the privacy and security of data subjects. Medical devices and GDPR cross paths in many areas.
-

Our employees' medtech resolutions include great ideas. What are your improvement plans? We'd love to hear and share them.
-
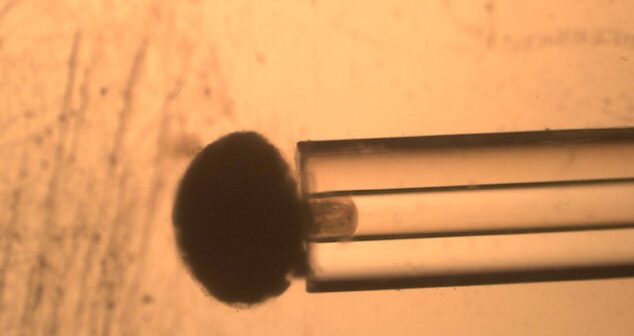
The technique of Micropipette Aspiration is an inexpensive way to measure the viscoelastic properties of cells.
-

Learn how the blockchain technology behind Bitcoin will become healthcare’s underlying technology to focus around patients and diseases.
-
Design control shouldn’t inhibit creativity; it should enable it by providing a safety net in the form of a system to fall back on.
-

Top 10 StarFish Medical blogs of 2017 feature a mix of regulatory, engineering, and practical information and advice for all levels of expertise.