Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
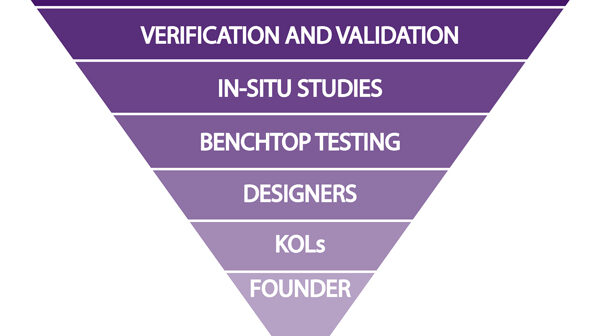
Balancing cost and feedback in medical device development is about when, from whom, and how much weight to give clinical input.
-
DC bias effects on capacitance can be significant: what can we do about it?
-

MLCCs have many attractive features: low ESR, good capacitance-to-volume ratio, relatively low leakage, non-polarity, and low cost.
-

11 Lessons learned from medical device project implementations cover a range of topics from regulatory and quality to development.
-

Computer games and medical device design both begin conceptually in a somewhat similar space – a point where reality and science fiction meet.
-

Ways to improve medical device user experience and adoption based on initiatives related to Electronic Health Record.
-
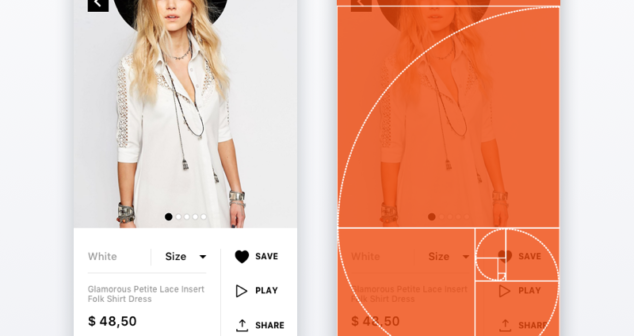
Designing the look and feel of the medical device user interface includes which shade of red to use for your user interface.
-
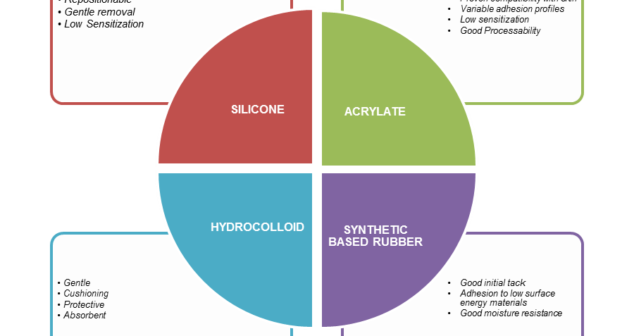
Successful wearable medical devices and adhesive selection require solid understanding of the basics of adhesives along with Do's and Don'ts.
-
The don’ts of developing wearable medical devices in order to prevent any costly fixes that may arise later in development.