Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
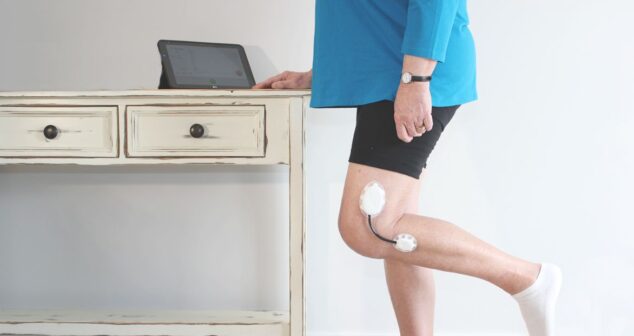
First of three blogs series offers overview and explains the dos of skin-worn wearable medical device design and development.
-
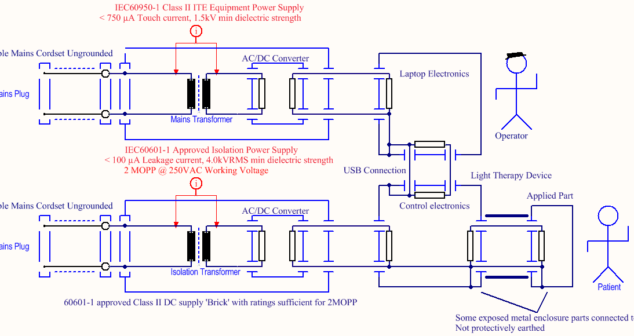
Creating an electrical insulation diagram early on helps to choose the right parts and is required by IEC 60601-01.
-

Overview of FDA Regulation of E-cigarettes Deeming Tobacco Products to be subject to the Federal Food, Drug and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act.
-

Digital Health communication technology cost and deployability for medical devices features a discussion of StarFish engineers
-

Implications of Device Generated Data using medical devices capable of data generation and uploading into digital health.
-

Electrical Engineer's overview of IEC 60601-1 covering Terminology & Definitions through Annexes. Part 2 of 2 blogs on the topic.
-
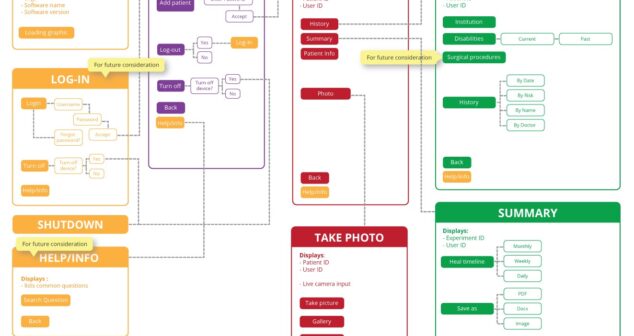
Medical Device UX design process – Design & Testing discusses Information architecture, Wireframing, and Formative Evaluation.
-

EE's overview of IEC 60601-1 Scope and Normative References, one of the more important standards that apply during medical device development.
-
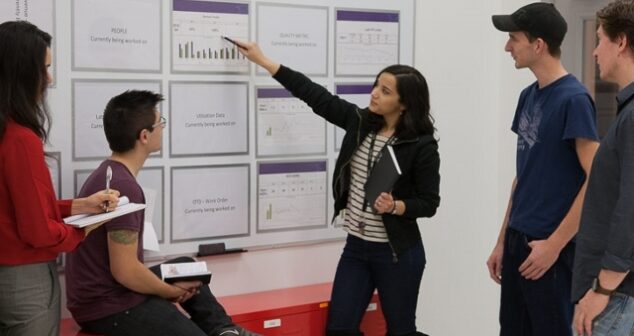
13 ways a PMO (Project Management Officer) manages their responsibility for all of the actions a company does in project management.