Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

What Attracts Employees StarFish Medical? Employees share expectations prior to joining with experiences being part of StarFish.
-
Overview of the FDA action plan for AI/ML in software as a medical device (SaMD) and list of best practices.
-

2021 top medical device commercialization videos cover 2021 updates, optimizing founder value, manufacturing for NPI, IVD insights. and more.
-

2021 “most read” list: Electrical engineering and regulatory articles tie for the most popular blog topics.
-
Medical Device New Product Introduction (NPI) & Commercialization lessons for medical device strategy, design, development, and NPI success.
-
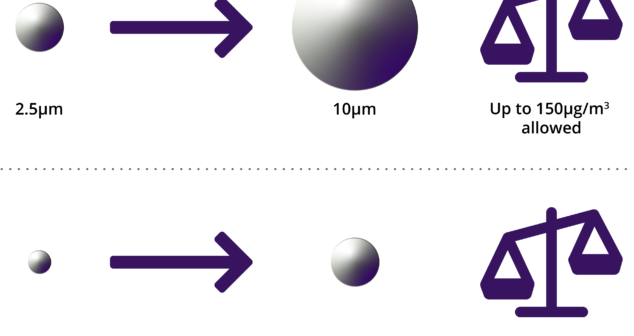
Pre-Testing for ISO 18562-2:2017; how to approach in-house pre-testing and add confidence to 18562:2-2017 formal testing.
-

2021 Update on Health Canada's medical device regulatory development during the COVID-19 pandemic and lessons learned from the experience.
-

Listening Strategy for Startups offers a three-pronged approach to stay competitive within the startup ecosystem.
-

Outlines how medical device designers can perform in-house Medical Device Drop Test per IEC 60601-1 before third party testing.