Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
Staffing a Medical Device Start-Up: shortlist of job descriptions required to develop a complex, novel medical device.
-
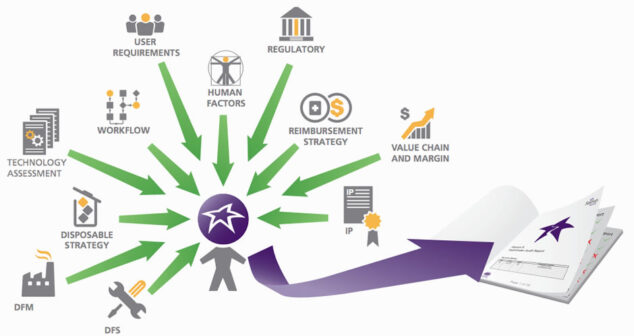
Medical Device Pathfinder process for Intellectual Property, Consumables Strategy, Value Chain and Margin, and Reimbursement.