Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

6 benefits mean the FDA program for breakthrough medical devices may be your best regulatory option for novel medical devices.
-

FDA's Early Feasibility Studies Program (EFS) is a great option in early stage development looking to advance your program by gathering data.
-

Proper research and communication with authorities in each country of interest will make Medical Device International Regulations smoother to navigate.
-

ISO/IEC 17025:2017 includes many changes. Three points to keep in mind: more options, involvement of risk, updates in current technology.
-

Why design control is required for medical devices and is superfluous in the consumer goods design with advantages and disadvantages.
-
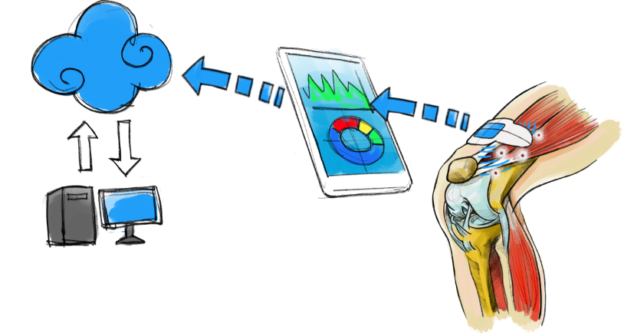
GDPR is designed to enhance the privacy and security of data subjects. Medical devices and GDPR cross paths in many areas.
-
Design control shouldn’t inhibit creativity; it should enable it by providing a safety net in the form of a system to fall back on.
-

StarFish Medical videos help viewers learn medical device design, development and manufacture insights and tips from the people at StarFish.
-

RoHS and REACH are two regulations that requires compliance if a company intends to sell in the European Union.