Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Interference Testing, Strategy, Detection Case Study examines POC medical devices assay verification guideline CLSI EP07-A2.
-
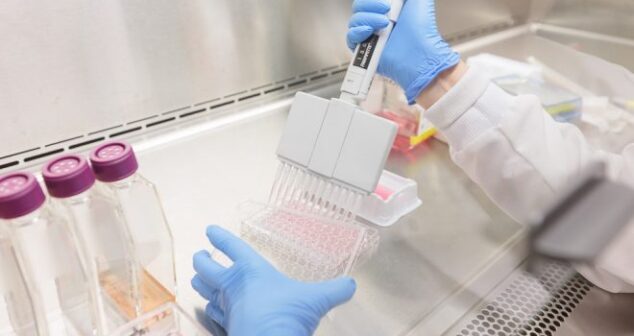
Simulated Fluids for POCT Development enable testing using a liquid that mimics the critical properties of the desired clinical sample.
-

Testing Medical Devices in a Biolab? Pre-planning is required for specialized tests, potentially dangerous microorganisms, tissues and biological fluids.
-
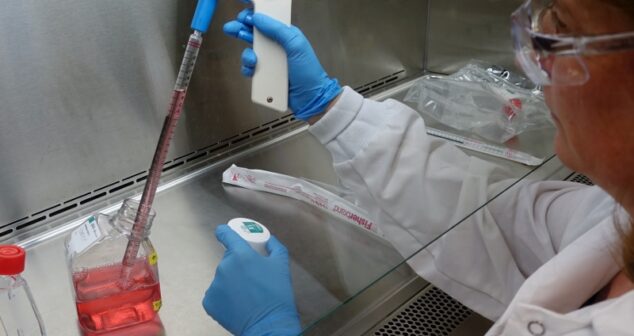
Overview of Simulated Fluids (SF) includes 5 of the most common simulated biological fluid recipes to aid in medical device development.
-

Simulated Fluids help avoid high costs of animal trials and exposure risks to infectious biological for early stage medical device design.