Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Rules for Medical Device Entrepreneurs from Paul Geyer captured during medical device executive panel discussion at #MDPLAYBOOK.
-
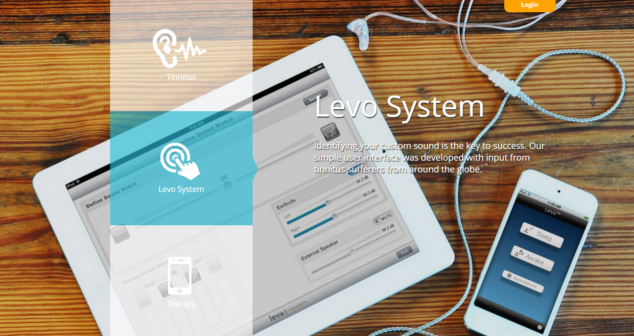
Digital Health & medical device regulatory and technical implications: HIPAA, Mobile Medical App (MMA), Medical Device Data System (MDDS), and app stores.
-

Digital Health Advantages and Disadvantages describes numerous advantages and motivations for medical devices to engage with Digital Health.
-

Results of StarFish client satisfaction survey with a large group that included customers and prospects using one word descriptions.