Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
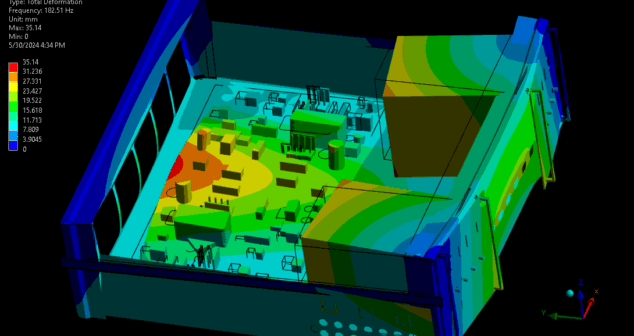
The impact of vibration on medical devices can be understood through a combination of vibration analysis and testing. This article reviews medical devices most susceptible to vibration, provides basic theory and methods of vibration analysis, and summarizes the benefits of using modal analysis when developing medical devices.
-

Medical Device Case study example where "dig deeper" – IEC 60601 saved the author from potential problems later down the road.
-

Electrical Engineer's overview of IEC 60601-1 covering Terminology & Definitions through Annexes. Part 2 of 2 blogs on the topic.
-

EE's overview of IEC 60601-1 Scope and Normative References, one of the more important standards that apply during medical device development.