Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Developing medical devices with software involves a wide-ranging set of unique challenges. Our software engineering team offer advice on offered advice on software in medical devices, FDA, Software as a Medical Device (SaMD), risks, cybersecurity, and Open Source software.
-

QNX Medical Device Bootscreen tips for the popular micro-kernel OS designed for embedded systems and safety critical hardware.
-
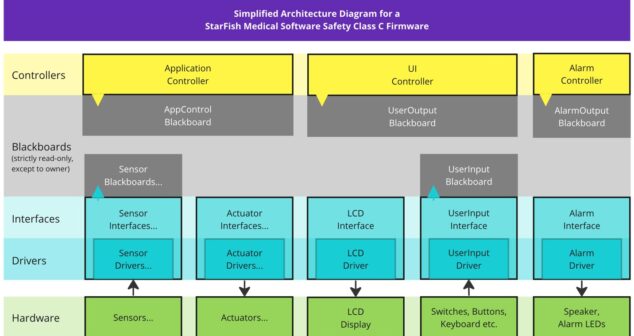
How to develop Class C Firmware for medical devices and implement Segregation in compliance with the IEC 62304 Standard.
-
Medical device software is very diverse. In all this variety, one technology is ubiquitous: Git! Three eye-openers which help developers use Git better.