Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
Continuous Integration (CI) firmware will help medical device software developers show regulators that their code is of high quality.
-
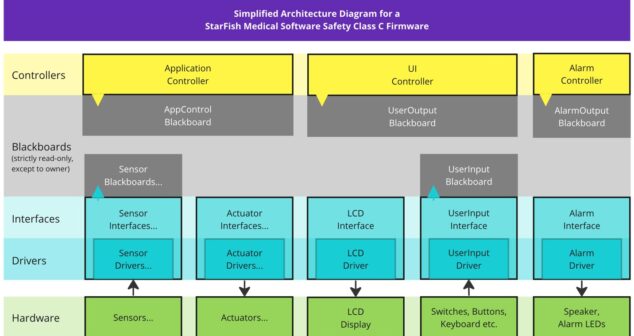
How to develop Class C Firmware for medical devices and implement Segregation in compliance with the IEC 62304 Standard.