Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
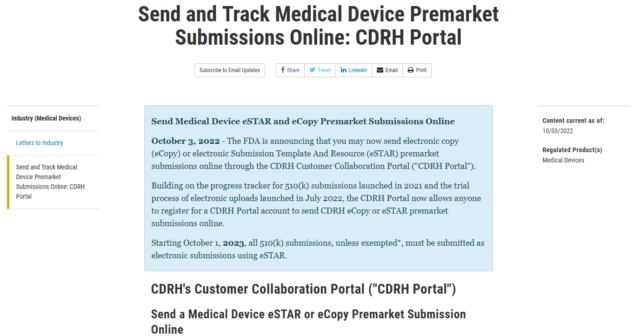
Overview of eSTAR, a joint Health Canada and FDA program streamlining medical device submissions with info on how to apply for the program.
-

2021 Update on Health Canada's medical device regulatory development during the COVID-19 pandemic and lessons learned from the experience.
-

Health Canada improvements: Getting new devices to market, strengthen monitoring and follow-up and provide more information to Canadians.
-

Health Canada has announced online publication of Regulatory Decision Summaries (RDS) and a list of decisions under review.