
How to benefit from FDA ASCA (Accreditation Scheme for Conformity Assessment) Pilot Program
FDA launched their pilot ASCA (Accreditation Scheme for Conformity Assessment) Program on September 25, 2020. The intent for the ASCA program is to utilize accredited third-party testing laboratories to assess premarket applicants’ declarations of conformity to consensus standards for safety, performance and biocompatibility. The final guidance outlining the accreditation scheme for the ASCA pilot program can be found here.
The ASCA program is voluntary and free for participation for manufacturers. It is intended to enhance product reviewers’ and device manufacturers’ confidence in medical device testing. This should decrease FDA requests for additional information regarding testing methodologies when a premarket submission includes declarations of conformity to an FDA-recognized consensus standard eligible for inclusion in the ASCA Pilot. Ultimately, the ASCA Pilot is intended to help the FDA ensure patients have timely and continued access to safe, effective, and high-quality medical devices.
Utilizing accredited testing labs should help make premarket reviews of medical devices and In Vitro Devices (IVD) more consistent and efficient. FDA is launching the ASCA Pilot hoping that it will decrease the burden for review of individual premarket submissions when manufacturers use testing completed by ASCA-accredited testing laboratories.
How does the ASCA Pilot work?
Here is an FDA flow diagram of the process:
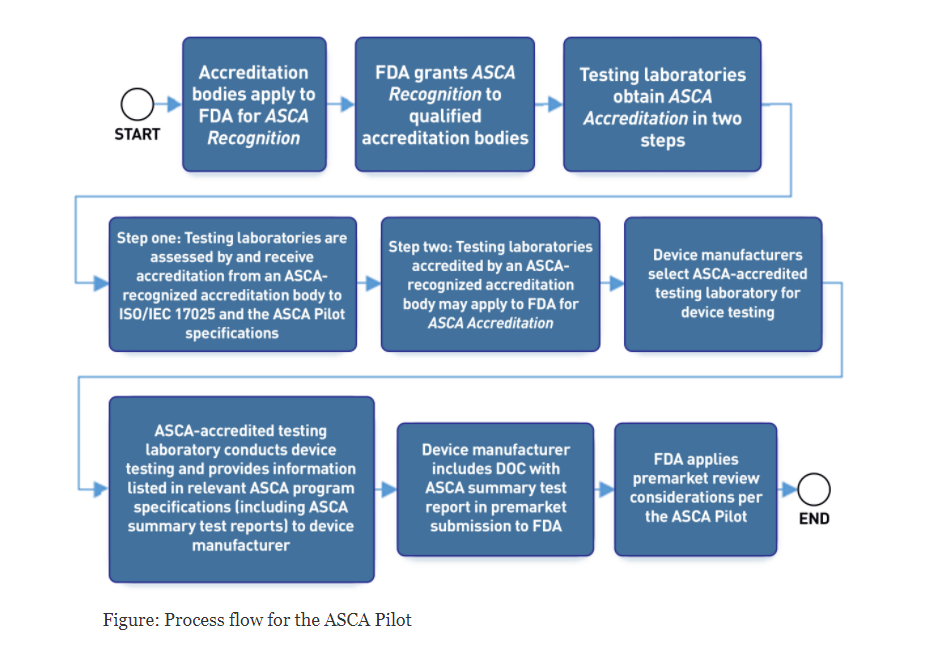
- Accreditation bodies may apply to the FDA for ASCA Recognition. FDA grants recognition to organizations who meet the ASCA program requirements.
- Testing laboratories apply to the FDA for ASCA Accreditationin a two-step process including an assessment by an ASCA-recognized accreditation body to ISO/IEC 17025:2017: General requirements for the competence of testing and calibration laboratories, and the additional ASCA Pilot specifications.
- FDA reviews the application. Organizations who meet the ASCA Pilot qualifications specified in the ASCA Pilot program guidance are granted ASCA accreditation.
- A device manufacturer may choose to use an ASCA-accredited testing laboratory to conduct testing for premarket submissions to the FDA.
There is no limit to the number of accreditation bodies who may receive ASCA Recognition. When the ASCA Recognition is granted to an accreditation body, the FDA provides a renewable expiration date for ASCA Recognition. Currently there are five accreditation bodies recognized by FDA. Their scope covers IEC 60601, ISO 80601 and ISO 10993 test methods. view the list of the for the ASCA program here.
ASCA-accredited testing laboratories are accredited for the ASCA Pilot using ISO/IEC 17025 and the ASCA program specifications. Because several ASCA program specifications for biological evaluation standards and test methods address the need for Good Laboratory Practice (GLP) compliance, ASCA-accredited testing labs need to be GLP compliant.
How does the program work for manufacturers?
Manufacturers can use an ASCA-accredited testing laboratory to conduct testing intended for inclusion in a premarket submission. At the time of premarket submission, the manufacturer is still required to submit a declaration of conformity with the necessary supplemental documentation, which in this case would be the ASCA summary test report and a justification of how the testing supports the premarket submission.
FDA has issued two standard-specific guidance documents providing examples for the declaration of conformity and the ASCA summary test reports. The first guidance addresses the requirements for IEC 60601 and ISO 80601 testing and can be found here. The second guidance addresses the requirements for biocompatibility testing and can be found here.
No Requirements to Apply:
Manufacturers don’t need to apply to participate in the ASCA program. They simply need to get the required testing performed by an ASCA-accredited lab. If a manufacturer prefers to perform the testing in house, they can apply to have in-house labs located in the USA certified through the process described above.
Eligible Devices and Submissions:
All devices are eligible for ASCA-accredited testing, including combination products.
FDA’s Review of ASCA Accredited Testing:
All testing by ASCA-accredited laboratories will be performed using methodologies consistent with FDA-recognized consensus standards and methodologies. FDA will rely on the results for the purpose of premarket review when the testing is accompanied by a declaration of conformity and ASCA summary report, and the standard and test methods used are within the scope of the lab’s ASCA Accreditation at the time of testing.
Manufacturer’s Responsibilities for Premarket Submissions:
All relevant information for a premarket submission should be provided. If testing is performed by ASCA-accredited lab, the manufacturer is still responsible for providing justification on how the data supports the premarket submission. Manufacturers don’t
In conclusion, the goal of the new ASCA Pilot Program is to bring medical devices to patients and users more efficiently by using ASCA-accredited labs. This new process will hopefully decrease FDA requests for additional information and streamline and expedite the review process.
Virginia Anastassova, RAC, is the Regulatory Affairs Manager/ Senior QA Specialist at StarFish Medical. She brings extensive experience in quality management and regulatory affairs to our clients.
Illustration: FDA Guidance
