Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
The simplest and least expensive way to train users of medical devices is to ask them study the Instructions for Use (IFU) beforehand.
-

User Experience (UX), Human Factors (HF), and Industrial Design (ID) each have a major impact on the success of new medical devices. Their influence is especially important during product definition and early phases of device development.
-

Medical device drop testing helps ensure that products and packaging survive real-world handling. We demonstrate in-house drop testing on an actual device and its packaging using a custom-built drop tester.
-

A structured, well-documented design review process is a critical component of successful product development, particularly in the medical device industry.
-

The human eye is an extremely delicate organ, often prone to irritation, dryness and various diseases, such as glaucoma, cataracts, keratoconus, age-related macular degeneration, and many others. These ocular clinical conditions also affect patients’ quality of life.
-
Exploration of drug-device combination therapies that are transforming the treatment of Parkinson’s, epilepsy, depression, and brain cancer.
-
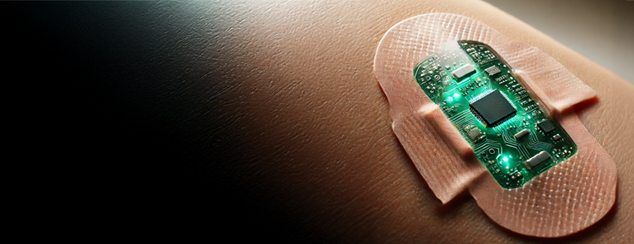
With the recent developments and seemingly ubiquitous nature of real time glucose monitoring and availability of smart wearable tech, the development of a theranostic band-aid seems inevitable. But how practical would this be? Is there a Theranostic wound dressings market?
-

What are the real costs of developing a medical device? In this episode of Bio Break, Nick and Joris dive into one of the most frequently asked questions they hear from clients: How much does it cost to develop a medical device?
-

Predicting the trends of a new year is always interesting and a bit unpredictable. We asked our medical device design and development professionals to submit their most interesting medtech trends for 2025 and the reasoning behind their prediction. The results were surprisingly focused on two major trends: Home Healthcare and Wearable Devices. Within these categories, several technologies were identified including edge computing, IoT, and connected devices. In no particular ranking, here are our 2025 predictions: